Introduction
Pneumonia is a common type of lung infection in one or both lungs that can lead to serious complications including lung abscesses, respiratory failure, and sepsis [1]. Community-acquired bacterial pneumonia (CABP) is pneumonia caused by a bacterial pathogen – usually Streptococcus pneumoniae – in a community setting [1]. In 2019, influenza and pneumonia were the ninth leading cause of death in the USA with 49,783 deaths [Reference Xu, Murphy, Kochanek and Arias2]. Annually, over one million hospitalizations and 540,000 emergency department visits in the USA are attributed to pneumonia [Reference McLaughlin, Khan, Thoburn, Isturiz and Swerdlow3]. For patients aged 65 years and older, rates of hospitalized CABP averaged 1899 per 100,000 person-years (PY) [Reference McLaughlin, Khan, Thoburn, Isturiz and Swerdlow3]. For patients younger than 65 years of age, rates of hospitalized CABP averaged 285 per 100,000 PYs [Reference McLaughlin, Khan, Thoburn, Isturiz and Swerdlow3]. Patients with CABP also create a substantial burden on hospital resources. For patients admitted to the hospital with CABP, mortality during hospitalization exceeds 6%, and the cost of treatment is approximately $17,736 per patient, with a hospital length of stay (LOS) averaging 5.7 days [Reference Ramirez, Wiemken and Peyrani4,Reference Divino, Schranz, Early, Shah, Jiang and DeKoven5].
To decrease the burden associated with CABP, the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) jointly published guidelines for the management of CABP in 2019 [Reference Metlay, Waterer and Long6]. These recommendations were developed by a multidisciplinary panel conducting systematic reviews of relevant literature and clinical trials [Reference Metlay, Waterer and Long6]. Guideline-concordant antibiotic therapy has been associated with shorter time to clinical stability [Reference Frei, Restrepo, Mortensen and Burgess7], shorter time to switch therapy [Reference Frei, Restrepo, Mortensen and Burgess7], reduced hospital LOS [Reference Frei, Restrepo, Mortensen and Burgess7,Reference Frei, Jaso and Mortensen8], and improved patient survival in patients with CABP admitted to the hospital [Reference Frei, Restrepo, Mortensen and Burgess7].
Investigations into racial and/or ethnic disparities in prescribing patterns of guideline-concordant antibiotic therapy for CABP have been sparse since the recommendations were published in 2019. In prior reports, both in the general inpatient ward and intensive care settings, Black and Non-Hispanic White (NHW) patients have been observed to be equally as likely to receive ATS/IDSA guideline-concordant antibiotic therapy [Reference Frei, Mortensen and Copeland9,Reference Hausmann, Ibrahim and Mehrotra10]. Additionally, 30-day mortality was similar between groups in the general inpatient ward but was found to be lower for Black patients vs. NHW patients when admitted to the intensive care unit (ICU) [Reference Frei, Mortensen and Copeland9]. Further, Hispanic and Asian patients had lower mortality than NHW or Black patients [Reference Mortensen, Cornell and Whittle11,Reference Haas, Dean, Hung and Rennie12]. However, Black and Hispanic patients were less likely to receive timely initiation (within four hours) of antibiotic therapy [Reference Frei, Mortensen and Copeland9–Reference Mortensen, Cornell and Whittle11], appropriate vaccination (pneumococcal and influenza) [Reference Hausmann, Ibrahim and Mehrotra10,Reference Mayr, Yende and D’Angelo14], and smoking cessation counseling [Reference Mayr, Yende and D’Angelo14]. These differences are seen more prominently between different hospitals than within the same hospital [Reference Frei, Mortensen and Copeland9,Reference Hausmann, Ibrahim and Mehrotra10,Reference Mayr, Yende and D’Angelo14]. There has been little evidence of race- or ethnicity-based differences in guideline-concordant prescribing for the management of CABP [Reference Frei, Mortensen and Copeland9,Reference Mortensen, Cornell and Whittle11]. Of note, guideline-concordant antibiotic prescribing for patients admitted to the hospital with CABP has not yet been evaluated in the All of Us cohort.
The All of Us Research Program is an effort led by the National Institutes of Health (NIH) to increase the rate of health-related research discoveries, further develop precision medicine, and analyze the impact of environment, lifestyle, genetics, and other determinants of health on disease and health [15]. To accomplish this, the All of Us Research Program is working to create a diverse health database that includes data from groups traditionally underrepresented in research by enrolling at least 1,000,000 people from the USA over the span of 10 years [15]. To date, 590,000+ participants have consented to be enrolled and 411,000+ participants have completed the first steps of the program [16]. The first steps of the program provide baseline data for each participant, and they include consenting, providing physical measurements, the donation of at least one biospecimen to the All of Us biobank, agreement to share electronic health records, and completion of the first three surveys [16]. The data are publicly available and open-sourced with access being stratified into three tiers to protect participant privacy (Public, Registered, and Controlled Tier) [17]. Data are collected through participant-provided information (PPI), electronic health records, biospecimens, and digital health devices (wearable sensors, apps, mobile phones, etc.) [15,16].
This research uses newly available All of Us data to examine guideline-concordant treatment of CABP. In this study, we identify whether disparities exist in guideline-concordant antibiotic prescribing among patients admitted to the hospital for CABP in the All of Us database by age-, race-, sex-, and ethnicity-based subgroups.
Methods
Regulatory and Ethics
All data collection, analysis, and dissemination were conducted in accordance with the All of Us Data User Code of Conduct and all policies therein. Institutional Review Board approval was obtained from University of Texas Health Science Center at San Antonio Institutional Review Board (IRB) and Office of Clinical Research (OCR) before beginning the study (Protocol number: 20220682EX).
Data Source and Study Population
We identified all adult patients with a diagnosis of pneumonia (SNOMED 233604007), or one of its descendent concepts (n = 21), after October 1, 2018, in the All of Us database using version 6 of the Curated Data Repository (CDR). Version 6 of the CDR (released June 2022) includes data collected between October 1, 2018, and January 1, 2022. The date used for screening is one year before the publication of the 2019 ATS/IDSA guidelines. This is done to account for the “date shifting” found in the All of Us database. This shift translates to all dates associated with a participant shifted back 1–365 days, but the shift is constant for each participant to preserve temporality of events [18]. The All of Us database collects both gender identity and sex assigned at birth using “The Basics” survey, which is PPI [19]. For this study, sex was determined using data from the sex assigned at birth question.
Inclusion and Exclusion Criteria
Patients included in this study were those who had a diagnosis of pneumonia present in their record and were prescribed antibiotics during the same encounter as the pneumonia diagnosis.
Patients were excluded for the following:
-
Patient must not have had a previous case of pneumonia less than 90 days prior to current case. For patients with multiple cases on record, there must have been an uninterrupted span of at least 90 days. If there was not an uninterrupted span of 90 days between cases, then the earliest visit was counted.
-
Patient must not have had parenteral antibiotics prescribed during an inpatient visit in the 90 days before the pneumonia encounter.
-
Patient must not have had a prior isolation of methicillin-resistant Staphylococcus aureus (MRSA).
-
Patient must not have had a prior isolation of Pseudomonas aeruginosa.
-
Pneumonia visit must have had a designation of inpatient or outpatient visit.
-
Encounter must not have been an ICU visit.
-
Patient must not have lived in a nursing facility in the 90 days before the pneumonia case.
-
The pneumonia case must not have been due to chronic, aspiration, hospital acquired, or ventilator acquired pneumonia.
Data Collection
Information was extracted from the All of Us database using the cohort builder tool and analyzed using the Jupyter notebook analysis environment inside of All of Us Researcher Workbench. Information was extracted using the cohort builder tool, concept sets for pneumonia, demographics, and antibiotic treatment received, and dataset builder tool. All tools used were found in the All of Us Researcher Workbench. The data access tier used was the Registered Tier, which contains individual-level data including electronic health records (EHRs), wearables, survey responses, and physical measurements.
Study Design
This is a retrospective cohort study of patients admitted to the hospital with pneumonia after October 1, 2018, through January 1, 2022, in the All of Us database (n = 1608). Patients were stratified into groups based on whether they received guideline-concordant or guideline-discordant antibiotic therapy as defined by the 2019 ATS/IDSA guidelines at any time during their admission [Reference Metlay, Waterer and Long6]. They were then further stratified into subgroups based on patient age [≤65 years (n = 994) vs. 65+ years (n = 614)], sex [female (n = 807) vs. male (n = 780)], race [Black (n = 560) vs. NHW (n = 592)], and ethnicity [Hispanic (n = 336) vs. NHW (n = 592)]. For the race subgroup, patients who did not identify as NHW or Black or did not indicate their race were categorized as Other. For the ethnicity subgroup, patient who did not identify as NHW or Hispanic or did not indicate their ethnicity were classified as Other. Percent of subgroups who received guideline-concordant antibiotic therapy was then compared using chi-square analysis. Then, when there were statistically significant differences (p < 0.05) between the subgroups, multivariate logistic regression models were created with subgroup as the independent variable, guideline-concordant therapy as the dependent variable, and divergent (significantly different) baseline characteristics as covariates. Specific medications prescribed were then cataloged, and percentage of subgroups who received the specific medications were compared using chi-square analysis.
Study Variables and End Points
Guideline-Concordant and -Discordant Definitions
The 2019 guidelines for empiric antibiotic treatment of bacterial pneumonia, as defined by the ATS/IDSA, were used to subset patients into two groups based on the antibiotics prescribed during the pneumonia visit. Patients who received β-Lactam (e.g., ampicillin + sulbactam, cefotaxime, ceftriaxone, or ceftaroline) and macrolide (azithromycin or clarithromycin) combination therapy, β-Lactam and doxycycline combination therapy, or respiratory fluoroquinolone (levofloxacin or moxifloxacin) monotherapy were considered to have been prescribed “guideline-concordant” antibiotics [Reference Metlay, Waterer and Long6]. We used the 2019 ATS/IDSA guidelines as our reference for what constitutes guideline-concordant antibiotic therapy, but these antibiotic recommendations have been consistent for several versions of the guidelines – well before 2019 – so all patient visits in this study occurred well after adoption of these antibiotic recommendations into evidence-based guidelines. Patients who received other regimens were considered to have received “guideline-discordant” antibiotics [Reference Metlay, Waterer and Long6].
Outcomes
The primary outcome of this study was the receipt of guideline-concordant antibiotic therapy as defined by the 2019 ATS/IDSA guidelines for the management of patients admitted to the hospital for CABP.
Statistical Analysis
Jupyter notebook inside the All of Us Researcher Workbench was the analysis environment used for all statistical comparisons. The alpha level for significance was 0.05. Patient age, the only numeric variable, was not normally distributed, so we reported median and interquartile range and used the Wilcoxon rank-sum test to compare patient age between groups. Dichotomous variables like baseline characteristics and prescribing practices based on age, sex, race, and ethnicity were compared using chi-square or Fischer’s exact tests. Odds ratios and 95% confidence intervals for the receipt of guideline-concordant antibiotic therapy were calculated as well. Then, for subgroups where there was a statistically significant difference in antibiotic prescribing between groups, multivariate logistic regression models were created with subgroup as the independent variable, guideline-concordant therapy as the dependent variable, and divergent baseline characteristics as covariates. Patients who chose skip (n = 84), prefer not to answer (n = 63), or don’t know (n = 10) for any of the categories were excluded from logistic regression of race and guideline-concordant prescribing (note: categories are not mutually exclusive). Patients who chose skip (n = 60), prefer not to answer (n = 23), or don’t know (n = 15) for any of the categories were excluded from logistic regression of ethnicity and guideline-concordant Prescribing (note: categories are not mutually exclusive).
Results
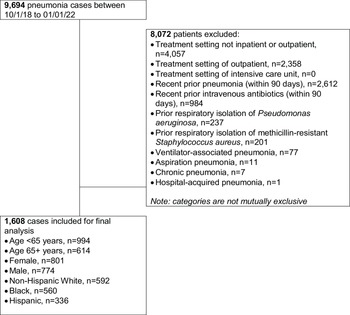
A total of 1608 patients with CABP met study inclusion/exclusion criteria (Fig. 1) and 45% (729/1608) received guideline-concordant antibiotic therapy. The guideline-concordant group was composed of patients treated with β-Lactam plus a macrolide (68%, 496/729), β-Lactam plus doxycycline (18%, 131/729), and fluoroquinolone monotherapy (33%, 241/729).

Figure 1. Study inclusion flow chart.
Patient Demographics
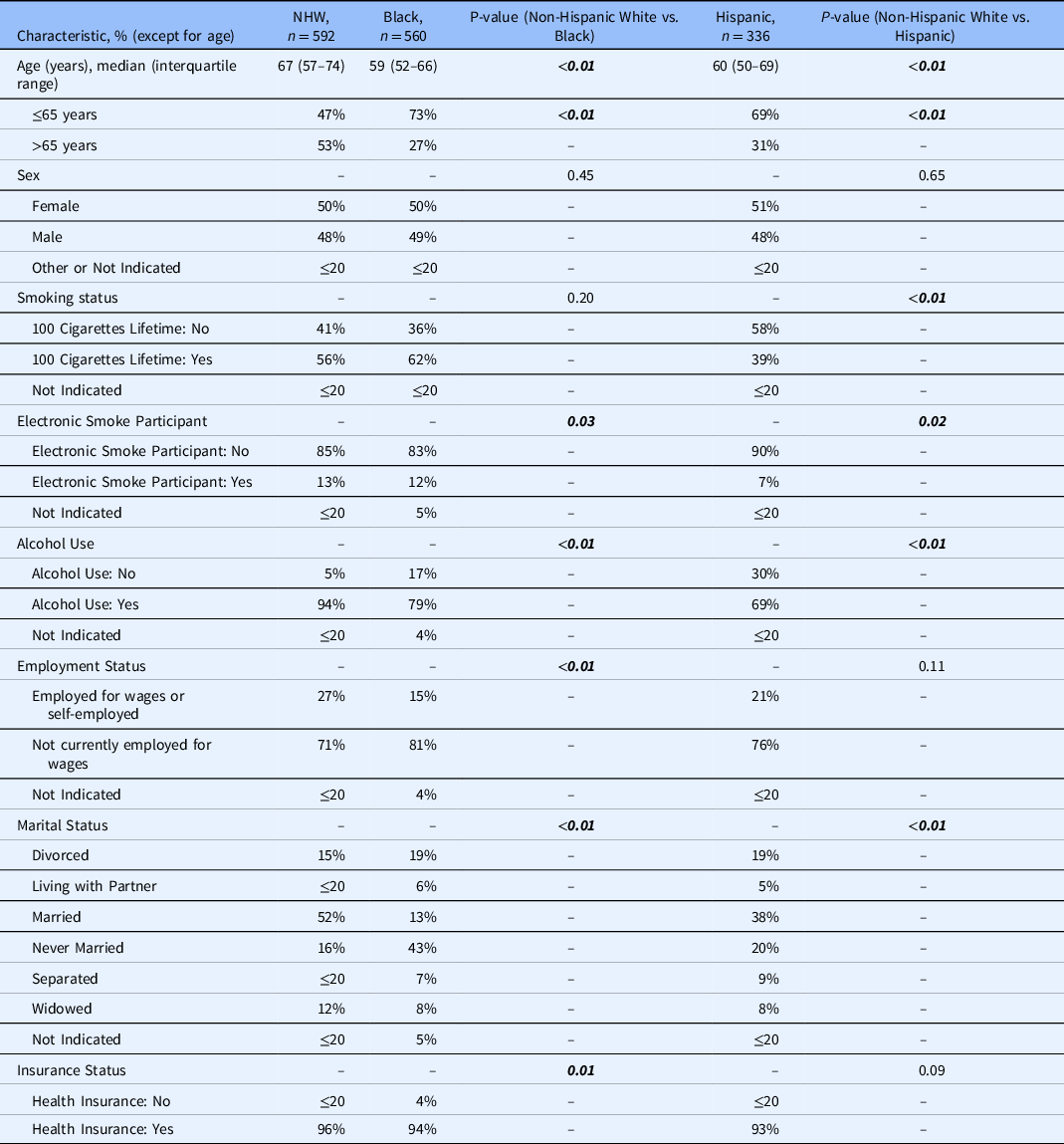
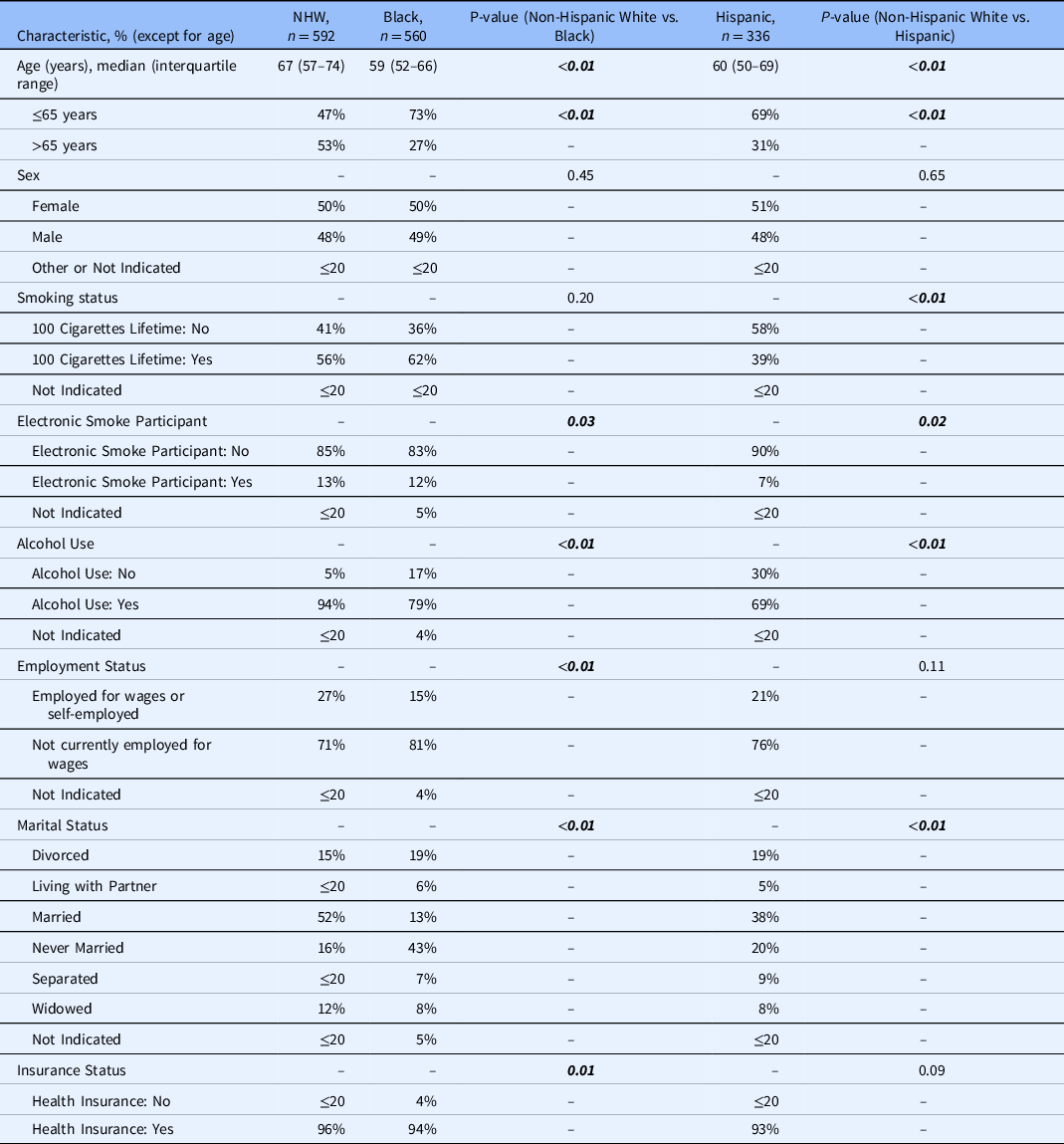
Table 1 represents demographics for patients admitted to the hospital with CABP and treated with guideline-concordant and -discordant antibiotic therapy in the All of Us database after October 1, 2018. There were significant differences in the percentage of patients prescribed guideline-concordant antibiotics between Black and NHW patients (p = 0.01) and between Hispanic and NHW patients (p = 0.02). The two groups were statistically similar regarding age, sex, smoking status, electronic smoking status, alcohol use, employment status, marital status, and insurance status.

* Bold italics indicate statistically significant findings.
** Counts of less than or equal to 20 are displayed as ≤20 to comply with the All of Us data and statistic dissemination policy [33].
Table 2 represents patient baseline characteristics by race and ethnicity. There were significant differences in age (years; p < 0.01), age (≤65 years vs. >65 years; p < 0.01) electronic smoking status (p = 0.03), alcohol use (p < 0.01), employment status (p < 0.01), marital status (p < 0.01), and insurance status between NHW and Black patients (p = 0.01). Between NHW and Hispanic patients, there were significant differences in age (p < 0.01), age (≤65 years vs. >65 years; p < 0.01), smoking status (p < 0.01), electronic smoking status (p = 0.02), alcohol use (p < 0.01), and marital status (p < 0.01).
Antibiotic Prescribing Patterns and Health Disparities
Table 3 represents guideline-concordant prescribing rates by age, sex, race, and ethnicity. Forty-seven percent of NHW patients (277/592), 38% of Black patients (216/560), and 54% of Hispanic patients (184/336) received guideline-concordant antibiotic therapy. There were no other significant differences based on race or ethnicity in medication choice for the guideline-concordant group.

* Bold italics indicate statistically significant findings.
** Counts of less than or equal to 20 are displayed as ≤20 to comply with the All of Us data and statistic dissemination policy [33].
Table 4 represents a breakdown of the medications prescribed for patients admitted to the hospital with CABP in the guideline-concordant group. Levofloxacin accounted for all fluoroquinolone use except for a subset of ≤20 patients who received moxifloxacin. Ceftriaxone was the only β-Lactam prescribed except for a subset of ≤20 patients who received ampicillin/sulbactam in combination with a macrolide. Azithromycin accounted for all macrolide use except for a subset of ≤20 patients who received clarithromycin. There were significant differences in prescribing rates of β-Lactams, specifically ceftriaxone, between Hispanic and NHW patients (p < 0.01). There were no other significant differences based on race or ethnicity in medication choice for the guideline-concordant group. Please refer to Supplemental Table 1 for a list of the most common guideline-discordant medications.

* Bold italics indicate statistically significant findings.
** Counts of less than or equal to 20 are displayed as ≤20 to comply with the All of Us data and statistic dissemination policy [33].
Table 5 represents the multivariate logistic regression analysis of guideline-concordant antibiotic prescribing and race. NHW vs. Black patients were associated with a 36% higher likelihood for receiving guideline-concordant treatment (adjusted OR, 1.36; 95% CI 1.02–1.81); age strata (≤65 vs. >65), electronic smoking use, alcohol use, employment status, marital status, and insurance status were used as covariates. Table 6 represents the multivariate logistic regression analysis of guideline-concordant antibiotic prescribing and ethnicity. NHW vs. Hispanic patients were associated with a 34% lower likelihood of receiving guideline-concordant antibiotics (aOR 0.66; 0.48–0.91); age strata [≤65 vs. >65], smoking status, electronic smoking use, alcohol use, employment status, and marital status were used as covariates.
Table 5. Logistic regression analysis of race and guideline-concordant antibiotic prescribing*,**,***

* Bold italics indicate statistically significant findings.
** Patients who chose Skip (n = 84), Prefer not to answer (n = 63), or Don't Know (n = 10) for any of the categories were excluded (total n = 124) from logistic regression of Race and Guideline-Concordant Prescribing. (Note: categories are not mutually exclusive).
*** The second variable listed is the reference group used in this model.
Table 6. Logistic regression analysis of ethnicity and guideline-concordant antibiotic prescribing*,**,***

* Bold italics indicate statistically significant findings.
** Patients who chose Skip (n = 60), Prefer not to answer (n = 23), or Don't Know (n = 15) for any of the categories were excluded (total n = 72) from logistic regression of Ethnicity and Guideline-Concordant Prescribing. (Note: categories are not mutually exclusive).
*** The second variable listed is the reference group used in this model.
Discussion
This study evaluated antibiotic prescribing for patients admitted to the hospital with CABP in the All of Us database. Previous studies have demonstrated that guideline-concordant antibiotic therapy is associated with improved clinical outcome for patients admitted to the hospital with CABP [Reference Metlay, Waterer and Long6,Reference Frei, Restrepo, Mortensen and Burgess7,Reference Frei, Attridge and Mortensen20,Reference Olson and Davis21]. Improved clinical outcomes include decreased LOS, shorter time to switch therapy, decreased mortality, and shorter time to clinical stability [Reference Metlay, Waterer and Long6–Reference Frei, Jaso and Mortensen8,Reference Frei, Attridge and Mortensen20,Reference Olson and Davis21]. Additionally, multiple studies have shown that guideline-concordant antibiotic therapy has been associated with lower total healthcare costs for ward patients hospitalized with CABP [Reference Frei, Jaso and Mortensen8,Reference Frei, Bell and Traugott22–Reference Han, Chen and Wang24]. Identifying potential variation and disparity is the first step in correcting them and therefore improving patient outcomes.
Less than half (46%, 729/1608) of patients admitted to the hospital for CABP in the All of Us database were prescribed guideline-concordant antibiotic therapy. These patient visits took place in October 2018 through January 2022, and as such, the treating physician would have access to previous iterations of the guidelines [Reference Mandell, Wunderink and Anzueto25]. One of the most significant changes between versions is the discontinuation of use of healthcare-associated pneumonia as a pneumonia category [Reference Metlay, Waterer and Long6,Reference Olson and Davis21]. This was done to shift emphasis to local epidemiology, previously validated risk factors, and antibiotic stewardship [Reference Metlay, Waterer and Long6,Reference Olson and Davis21]. In addition to this change, further evidence was gathered in favor of β-Lactam + macrolide combination therapy and fluoroquinolone monotherapy over β-Lactam monotherapy for the management of CABP [Reference Metlay, Waterer and Long6,Reference Olson and Davis21]. And finally, further evidence has strengthened the recommendation for β-Lactam + macrolide combination therapy over fluoroquinolone monotherapy, although both are still recommended [Reference Metlay, Waterer and Long6]. This is due in part to the potential symptoms of fluoroquinolone exposure, which include neuropathy, aortic ruptures and tears, tendinopathy, and C. difficile infection [Reference Olson and Davis21,26,Reference Brown, Khanafer, Daneman and Fisman27]. Individuals at higher risk for these complications include those with hypertension, a history of circulatory blockages or aneurisms, select genetic disorders, and the elderly [Reference Olson and Davis21,26–Reference Frei, Labreche and Attridge28]. Efficacy is similar between the two regimens; however, some studies have demonstrated that fluoroquinolone monotherapy leads to a reduces hospital LOS [Reference Frei, Mortensen and Copeland9,Reference Frei, Labreche and Attridge28–Reference Lodise, Kwa, Cosler, Gupta and Smith31]. β-Lactam plus macrolide combination therapy was the most prescribed guideline-concordant therapy (68%, 496/729), with ceftriaxone as the β-Lactam and azithromycin as the macrolide.
While only half of CABP cases received guideline-concordant treatment, the guideline-concordant and -discordant groups were similar in their sociodemographic characteristics except in race, ethnicity, and alcohol use. Across race and ethnicity-based subgroups, NHW patients were significantly different from Black and Hispanic patients regarding age, electronic smoking status, alcohol use, employment status, and marital status. The proportion of NHW patients older than 65 years was significantly higher than both the Black and Hispanic patients. The cause for the difference in guideline-concordant prescribing based on race and ethnicity is unclear; however, process variations between hospitals may be a contributing factor [Reference Mortensen, Cornell and Whittle11,Reference Mayr, Yende and D’Angelo14]. Previous studies have identified other care gaps related to inpatient management of CABP for Black patients such as increased time to initiation of antibiotic therapy [Reference Hausmann, Ibrahim and Mehrotra10,Reference Mortensen, Cornell and Whittle11,Reference Trent, George, Havranek, Ginde and Haukoos13,Reference Mayr, Yende and D’Angelo14], decreased vaccination counseling [Reference Hausmann, Ibrahim and Mehrotra10,Reference Mayr, Yende and D’Angelo14], and decreased smoking cessation counseling [Reference Hausmann, Ibrahim and Mehrotra10]. Further research into the clinical decision processes and workflows surrounding the treatment of CABP is needed reduce the barriers associated with guideline-concordant antibiotic prescription. Additionally, cultural competency/proficiency/humility training of healthcare workers might help mitigate these differences in prescribing practices. Further optimization of these processes could yield improved compliance with guidelines, patient outcomes, and cost and reduce CABP-associated hospital burden.
Because of its observational nature, this study has potential limitations. Investigators were unable to account for all risk factors for MRSA and P. aeruginosa infection due to lack of local epidemiological data. Additionally, investigators could not account for institution-level and individual variations that go into the decision-making process for antibiotic selection, including allergies to medication. This study has potential selection bias as patients were not randomized to treatment arms. The All of Us database does not currently contain enough patients (i.e., inadequate sample size) to investigate associations between antibiotic prescribing and health outcomes but does contain valuable information on prescribing patterns for various special populations. We can infer, based on previous research from Frei et al. [Reference Frei, Restrepo, Mortensen and Burgess7,Reference Frei, Jaso and Mortensen8], that patient populations that receive less guideline-concordant antibiotics are also less likely to experience positive health outcomes. Finally, there were no pneumonia patients from the ICU setting in the version of the All of Us database we used for this study. Even if there had been, the CABP guidelines recommend different antibiotics for patients admitted to the hospital ward and ICU, so we would have had to stratify patients by setting before determining if the antibiotics received were guideline-concordant or -discordant. Despite these limitations, the present work provides valuable information for the management of inpatients with CABP from a database including patients underrepresented in research.
Conclusions
In the All of Us database, Black patients admitted to the hospital with CABP were less likely to receive guideline-concordant antibiotic therapy when compared with NHW patients. In contrast, Hispanic patients admitted to the hospital with CABP were more likely to receive guideline-concordant antibiotic therapy than NHW patients. Identification of health disparities, such as differences in evidence-based antibiotic prescribing by race and ethnicity, are an important first step in combating health disparities.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cts.2023.567
Acknowledgments
The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. In addition, the All of Us Research Program would not be possible without the partnership of its participants [32].
Funding statement
This work was funded in part by NIH NCATS through Grant Award UL1TR002645 (Frei, Schmidt). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the authors’ affiliated institutions.
Competing interests
The authors have no conflicts of interest to declare.