Introduction
Sulfonylurea herbicides, discovered in the 1970s, bestowed a new standard in weed management. Chlorsulfuron and sulfometuron-methyl, first sold in 1981 and 1982, respectively, were the first commercialized compounds from this chemical family (Brown and Cotterman Reference Brown and Cotterman1994; Russell et. al. Reference Russell, Saladini and Lichner2002). In subsequent years, this chemical family has been intensively developed, and the agrochemical market currently offers more than 30 active ingredients (Herbicide Resistance Action Committee 2017). Compared with other chemical families of herbicides, sulfonylureas are applied at extremely low rates that are measured in several grams or several dozen grams per hectare. Moreover, sulfonylureas demonstrate excellent efficacy against many weed species, including difficult to manage weeds; high selectivity toward a wide range of cultivated plants; and low mammalian toxicity (Brown and Cotterman Reference Brown and Cotterman1994; Green and Cahill Reference Green and Cahill2003). Sulfonylurea herbicides inhibit acetolactate synthase, also called acetohydroxyacid synthase, an enzyme essential for biosynthesis of the branched-chain amino acids, isoleucine, leucine, and valine (Senseman et al. Reference Senseman2007). Herbicidal selectivity among plant species is based on differences in rates of metabolism and degradation of the active ingredient. Tolerant plants rapidly metabolize the herbicide (Russell et al. Reference Russell, Saladini and Lichner2002).
Efficacy of foliar-applied herbicides can be significantly enhanced by adjuvants added to spray solutions (Wang and Liu Reference Wang and Liu2007). Previous studies have shown the most effective additives to sulfonylurea herbicides are nonionic surfactants, crop oil concentrate, methylated vegetable oil, inorganic salts, and pH adjusters (Green and Cahill Reference Green and Cahill2003; Nalewaja et al. Reference Nalewaja, Praczyk and Matysiak1995a, Reference Nalewaja, Praczyk and Matysiak1995b). Adjuvants increase efficacy of foliar-applied herbicides by improving droplet spread on plant surfaces, facilitating foliar uptake and translocation of the active ingredient into plant tissues and thus reducing application rates of active ingredients (Beck et al. Reference Beck, Steurbaut and Spanoghe2012; Dong et al. Reference Dong, Zhu and Yang2015; Spanoghe et al. Reference Spanoghe, Schampheleire, Meeren and Steurbaut2007; Zabkiewicz Reference Zabkiewicz2000). In recent years, new types of adjuvants have attracted the attention of researchers. These adjuvants include dimethylethanolamine-based esterquats and ionic liquid-type Gemini surfactants (Castro et al. Reference Castro, Ojeda and Cirelli2014; Li et al. Reference Li, Yu, Chen, Li and Li2012).
In sustainable agriculture, a significant feature of plant protection is the use of environmentally safe materials that meet the principles of green chemistry. Such a trend is also currently emerging in the use of adjuvants. An adjuvant is identified as “green” when it is produced from natural, renewable raw materials and has a low effect on humans and the environment. Moreover, “green adjuvants” should not increase the toxicity of the active substance toward non-target organisms (Beck et al. Reference Beck, Steurbaut and Spanoghe2012).
Ionic liquids (ILs) are compounds that fulfill many principles of green chemistry. These compounds are characterized by melting temperatures below 100 C (Wasserscheid and Welton Reference Wasserscheid and Welton2008) and present unique properties directly linked to their structures. The proper combination of cation–anion coupling allows synthesis of ILs for a variety of different purposes. ILs are effective as green solvents (Hajipour and Refiee Reference Hajipour and Refiee2015) and electrolytes (Galiński et al. Reference Galiński, Lewandowski and Stępniak2006). Domagk was the first researcher to report the biological activity of ILs (quaternary ammonium salts) (Domagk Reference Domagk1935). Since then, a number of antimicrobial, antifungal, and antitumor ILs have been described (Messali Reference Messali2015; Pernak et al. Reference Pernak, Sobaszkiewicz and Mirska2003; Shamshina and Rogers Reference Shamshina and Rogers2014; Zakrewsky et al. Reference Zakrewsky, Lovejoy, Kern, Miller, Le, Nagy, Goumas, Iyer, Del Sesto, Koppisch, Fox and Mitragotri2014). However, applications of bioactive ILs are not limited to medical purposes. ILs also increase efficacies of agricultural fungicides (Pernak et al. Reference Pernak, Markiewicz, Łęgosz, Walkiewicz, Gwiazdowski and Praczyk2015b) and herbicides (Cojocaru et al. Reference Cojocaru, Shamshina, Gurau, Syguda, Praczyk, Pernak and Rogers2013; Pernak et al. Reference Pernak, Syguda, Janiszewska, Materna and Praczyk2011, Reference Pernak, Niemczak, Zakrocka and Praczyk2013b). In addition, ILs express antifeedant activity toward stored product pests, thus protecting harvested crops during storage (Pernak et al. Reference Pernak, Wasiński, Praczyk, Nawrot, Cieniecka-Rosłonkiewicz, Walkiewicz and Materna2012, Reference Pernak, Nawrot, Kot, Markiewicz and Niemczak2013a, Reference Pernak, Łęgosz, Walkiewicz, Klejdysz, Borkowski and Chrzanowski2015a). Recently, a new solution of ILs called double salt herbicidal ionic liquids was released (Choudhary et al. Reference Choudhary, Pernak, Shamshina, Niemczak, Giszter, Chrzanowski, Praczyk, Marcinkowska, Cojocaru and Rogers2017). ILs can be synthesized from natural products (Klejdysz et al. Reference Klejdysz, Łęgosz, Czuryszkiewicz, Czerniak and Pernak2016; Pernak et al. Reference Pernak, Łęgosz, Walkiewicz, Klejdysz, Borkowski and Chrzanowski2015a), leading to environmentally friendly, readily biodegradable products with high biological activity. Choline soaps have been described as ecofriendly compounds with high surface activity (Klein et al. Reference Klein, Touraud and Kunz2008, Reference Klein, Muller, Kraus, Brunner, Estrine, Touraud, Heilmann, Kellermeier and Kunz2013; Kunz et al. Reference Kunz, Maurer, Klein, Touraud, Rengstl, Harrar, Dengler and Zech2011). In this study, choline-based ILs with vegetable oils (rapeseed and coconut oil) as sources of anions are synthesized and then tested as effective and ecofriendly adjuvants for sulfonylurea herbicides.
Materials and Methods
Substrates, Herbicides, and Adjuvants Used
Choline hydroxide (46% in water), pelargonic acid (99%), glyceryl tristearate (technical), glyceryl trioleate (technical), canola oil, and coconut oil were purchased from Sigma-Aldrich (Poznan, Poland) and used without purification. Potassium hydroxide and solvents were purchased from Avantor (Gliwice, Poland) and used as obtained.
The following herbicides were used in biological tests: metsulfuron-methyl (Galmet 20 SG, P.U.H. Chemirol, Mogilno, Poland, 20% ai), iodosulfuron-methyl-sodium (Huzar 05 WG, Bayer CropScience S.A., Lyon, France, 5% ai), and tribenuron-methyl (Granstar 75 WG, DuPont International Operations Sarl., Geneva, Switzerland, 75% ai). Actirob® 842 EC (Bayer, Warsaw, Poland, rapeseed oil methyl ester, 733 g L−1) was used as an internal standard for tank-mixed adjuvants in the greenhouse studies.
Syntheses
Progress and conditions of all reactions were monitored using a Mettler-Toledo EasyMax 102 system (Mettler-Toledo, Greifensee, Switzerland).
Synthesis of Choline Nonanoate (1)
A 0.05 M aliquot of choline hydroxide (46% in water) was placed in a round-bottom flask and neutralized with a stoichiometric amount of pelargonic acid (99%). Reagents were mixed for 15 min, and then 15 ml of diethyl ether was added to remove excess pelargonic acid. After phase separation, the aqueous layer was isolated, and water was evaporated under vacuum. The obtained product was dried under vacuum at 60 C for 8 h.
Synthesis of Choline ILs from Triglycerides (2–5)
All reactions were completed according to a previously described procedure (Pernak et al. Reference Pernak, Łęgosz, Walkiewicz, Klejdysz, Borkowski and Chrzanowski2015a). A 0.03 M aliquot of choline hydroxide was mixed with 20 ml of 2-propanol in a round-bottom flask, and then 0.01 M of triglyceride or vegetable oil was added. Reagents were heated under reflux until the pH of the system remained constant. The solvent was then evaporated under reduced pressure at 60 C, after which flask contents were dissolved in 15 ml of distilled water, placed in a separator, and washed three times with 10 ml of diethyl ether to remove unreacted triglyceride. After phase separation, the aqueous layer was isolated, and water was evaporated under vacuum. In the final step, the products were dried under reduced pressure (10 kPa) at 60 C for 24 h.
Nuclear Magnetic Resonance Analysis
The NMR spectra were recorded using a Mercury Gemini 300 spectrometer (Varian, Cambridge, UK) with tetramethylsilane as the internal standard operating at 300 MHz for 1H NMR spectra and 75 MHz for 13C NMR spectra.
Choline nonanoate (1). 1H NMR (300 MHz, CDCl3): δ 0.87 (t, 3H), 1.25–1.31 (m, 10H), 1.48–1.51 (m, 2H), 2.04–2.09 (m, 2H), 3.23 (s, 9H), 3.51–3.54 (m, 2H), 3.97–3.98 (m, 2H); 13C NMR (75 Hz, CDCl3): δ 13.9, 22.6, 26.8, 29.4, 29.6, 29.9, 31.8, 38.5, 54.0, 55.7, 67.8, 180.4.
Choline stearate (2). 1H NMR (300 MHz, CDCl3): δ 0.88 (t, 3H), 1.26–1.32 (m, 28H), 1.51–1.55 (m, 2H), 2.08–2.12 (t, 2H), 3.28 (s, 9H), 3.59–3.61 (t, 2H), 4.01–4.03 (t, 2H); 13C NMR (75 Hz, CDCl3): δ 13.9, 21.7, 22.5, 24.9, 25.2, 26.8, 29.0, 29.5, 29.6, 31.8, 38.6, 55.8, 63.5, 68.0, 72.9, 180.3.
Choline oleate (3). 1H NMR (300 MHz, CDCl3): δ 0.88 (t, 3H), 1.23–1.32 (m, 20H), 1.50–1.54 (m, 2H), 1.98–2.01 (m, 4H), 2.07–2.10 (m, 2H), 3.24 (s, 9H), 3.53–3.57 (m, 2H), 3.98–4.01 (m, 2H), 5.32–5.35 (m, 2H); 13C NMR (75 Hz, CDCl3): δ 14.0, 21.7, 22.6, 25.2, 26.8, 27.2, 29.2, 31.8, 38.6, 54.1, 55.8, 63.3, 37.9, 72.7, 122.6, 180.7.
Choline fatty acid anions isolated from canola oil, abbreviated as choline canolate (4). 1H NMR (300 MHz, CDCl3): δ 0.88 (t, 3H), 1.23–1.35 (m, 18H), 1.50–1.54 (m, 2H), 1.98–2.03 (m, 4H), 2.07–2.10 (m, 2H), 3.25 (s, 9H), 3.55–3.57 (m, 2H), 4.00–4.02 (m, 2H), 5.32–5.35 (m, 2H); 13C NMR (75 Hz, CDCl3): δ 14.0, 21.7, 22.5, 26.9, 27.1, 29.2, 31.8, 38.6, 54.1, 55.8, 63.3, 67.9, 72.8, 127.8, 129.8, 180.5.
Choline fatty acid anions isolated from coconut oil, abbreviated as choline cocoate (5). 1H NMR (300 MHz, CDCl3): δ 0.88 (t, 3H), 1.24–1.31 (m, 16H), 1.49–1.53 (m, 2H), 2.05–2.10 (m, 2H), 3.25 (s, 9H), 3.53–3.57 (m, 2H), 3.97–4.01 (m, 2H); 13C NMR (75 Hz, CDCl3): δ 14.0, 21.7, 22.5, 25.2, 26.8, 29.7, 31.8, 38.6, 54.1, 55.8, 63.3, 67.9, 72.8, 127.7, 129.6, 180.5.
Solubility
Solubilities of the prepared ILs were determined according to Vogel’s Textbook of Practical Organic Chemistry (Furniss et al. Reference Furniss, Hannaford, PWG and Tatchell1989). Representative solvents were chosen and ranked by their Snyder polarity index value in descending order (water, 9.0; methanol, 6.6; dimethyl sulfoxide, 6.5; acetonitrile, 6.2; acetone, 5.1; ethyl acetate, 4.4; chloroform, 4.1; toluene, 2.3; hexane, 0.0). Tests were conducted at 20 C under ambient pressure. The term “complete solubility” refers to ILs (0.1 g of IL) that dissolve in 1 ml of solvent, while the term “limited solubility” refers to ILs (0.1 g of IL) that dissolve in 3 ml of solvent. The term “insoluble” refers to an IL (0.1 g) that does not dissolve in 3 ml of solvent.
Thermal Stability
Thermal transition temperatures were determined by differential scanning calorimetry using a Mettler-Toledo Stare DSC1 (Mettler-Toledo, Leicester, UK) unit under nitrogen. ILs (between 5 and 15 mg) were placed in aluminum pans and heated from 25 to 120 C at a heating rate of 10 C min−1, cooled with an intracooler at a cooling rate of 10 C min−1 to −100 C, and then heated again to 120 C. Thermogravimetric analysis was performed using a Mettler-Toledo Stare TGA/DSC1 unit (Mettler-Toledo, Leicester, UK) under nitrogen. ILs (between 2 and 10 mg) were placed in aluminum pans and heated from 30 to 450 C at a heating rate of 10 C min−1.
Surface Activity
Surface tensions were determined with the pendant-drop method (Berry et al. Reference Berry, Neeson, Dagastine, Chan and Tabor2015). The measuring device was a DSA 100 analyzer (Krüss, Germany; accuracy±0.01 mN m−1) set at 25 C (temperature controlled using a Fisherbrand FBH604 thermostatic bath [Fisher], with an accuracy of 0.1 C). Aqueous solutions of bio-ionic liquids (BILs) were freshly prepared using double-distilled water. The concentrations studied as initial samples were 0.01 mol L−1. Ten samples of each compound were prepared for surface-tension measurements by serial dilution. At each step, 8 ml of the previous dilution was add to 8 ml of water. Each of the prepared samples was hand shaken vigorously to disperse the compound and was used within 1 h after preparation. The principle of the pendant-drop method involves the formation of an axisymmetric drop at the tip of a syringe needle. A CCD camera records an image of the drop (3 ml), and surface tension (mN m−1) is calculated by analyzing the profile of the drop in accordance with the Laplace equation (Pernak et al. Reference Pernak, Syguda, Janiszewska, Materna and Praczyk2011). Values of the critical micelle concentration (CMC) and the surface tension at the CMC (γCMC) are determined based on analysis of linear regression of the intersection of the two lines drawn in the low- and high-concentration regions of the surface-tension curves (γCMC vs. log C curves) (Blesic et al. Reference Blesic, Marques, Plechkova, Seddon, Rebelo and Lopes2007). The contact angle of the sample above CMC is ascertained from the image of the drop on the examined surface (paraffin). After the actual drop shape and contact line are defined, the drop shape is adapted to fit a mathematical model used to calculate the contact angle. The most precise method to calculate this value is the Young–Laplace fitting (sessile-drop fitting), in which the entire drop contour is evaluated. After successful fitting of the Young–Laplace equation, the contact angle is determined as the slope of the contour line at the three-phase contact point (solid–liquid and liquid–air).
Herbicide Deposits
Droplets (2 μl) of spray solutions were applied manually with a micropipette to the upper surface of the first fully developed leaf of uniform oilseed rape (Brassica napus L.) plants at the 6-leaf stage. Immediately after the droplet dried, a 1 by 1 cm section of the treated leaf was removed, placed on aluminum stubs, and attached to a conductive carbon adhesive ribbon. Each sample was placed on a table, which was subsequently placed in a microscope chamber and cooled to −20 C. Pictures were recorded with a Hitachi S3000N scanning electron microscope under low vacuum (50 Pa) with a back-scattered electron detector.
Herbicidal Efficacy Evaluation
Seeds of B. napus, cornflower (Centaurea cyanus L.), common lambsquarters (Chenopodium album L.), and corn poppy (Papaver rhoeas L.) were planted into commercial peat-based potting material (Kronen, Cerekwica, Poland) in 0.5-L plastic pots. The resulting plants were grown in a greenhouse environment at 22±2 C, 60% relative humidity, with a day length of 16 h for the duration of the test. Plants were watered as needed and thinned to 5 plants pot−1 within 16 d after emergence. Herbicides were dispersed in water and applied using a moving sprayer (APORO, Poznan, Poland) with a XR TeeJet® 110 02 VP flat-fan nozzle (TeeJet Technologies, Wheaton, IL, USA) delivering 200 L ha−1 spray mixture at 200 kPa operating pressure to plants at the 4-leaf stage (BBCH 14). Herbicide treatments consisted of: (1) metsulfuron-methyl at 4 g ai ha−1, (2) iodosulfuron-methyl-sodium at 7.5 g ai ha−1, and (3) tribenuron-methyl at 15 g ai ha−1. Each herbicide was applied alone and with each choline fatty acid anion salt at 0.2% v/v, 0.4% v/v, or 0.8% v/v or with 0.75% v/v Actirob® 842 EC (17 total treatments for each herbicide). Plant responses were recorded by cutting plant shoots at surface level and recording the fresh weight of each harvested shoot separately (Sartorius BP 2000 S balance with 0.01 g precision; Sartorius, Göttingnen, Germany) at 3 wk after herbicide application. Means of fresh weights and percent reductions of fresh weights relative to fresh weights of untreated plants were compared to determine weed control of each treatment. Each experiment was conducted as a completely randomized design twice. Treatments containing metsulfuron-methyl on C. cyanus and tribenuron-methyl on B. napus were conducted in triplicate, while all other treatments consisted of four replications. Experimental variation was recorded as standard error of the mean (SEM). SEM values were calculated according to Equation 1:
where SEM is the standard error of the mean, s is the sample SD, and n is the number of samples.
Statistical Analyses
Each of the two series of greenhouse experiments was conducted in a completely randomized design. Each experiment for each herbicide consisted of 17 treatments that included five choline fatty acid anion salts (1–5) at three concentrations, a standard adjuvant (Actirob®), and a control with no added adjuvant (none). Each herbicide series contained three or four replications per treatment. Data were analyzed as a one-way ANOVA with a random series effect. A Fligner-Killeen median test of homogeneity of variances was conducted before ANOVA. Based on Fligner-Killeen analysis, some data were transformed with the Box-Cox transformation with the λ parameter. Tukey’s multiple post hoc test (α=0.05) was used to compare treatments. The program package R v. 3.0.2 was used for calculations (R Core Team 2015).
Results and Discussion
Synthesis and Characterization
Neutralization reactions were performed in the shortest time with the highest product yields. Hydrolysis reactions were completed with high yield after 10 to 15 min following the addition of triglycerides as shown in Figure 1.
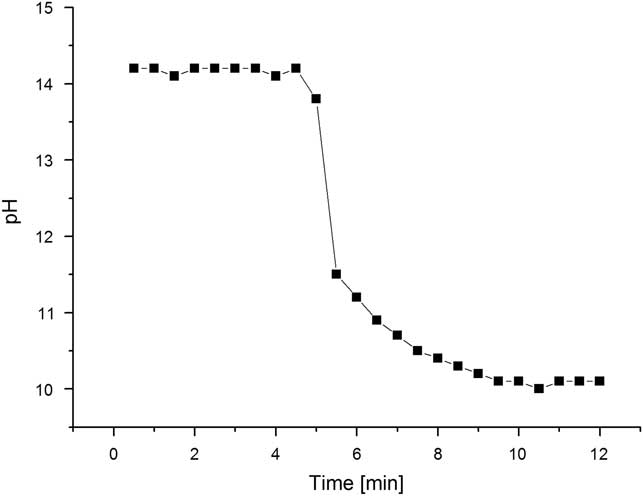
Figure 1 Changes of pH during synthesis of choline stearate (2).
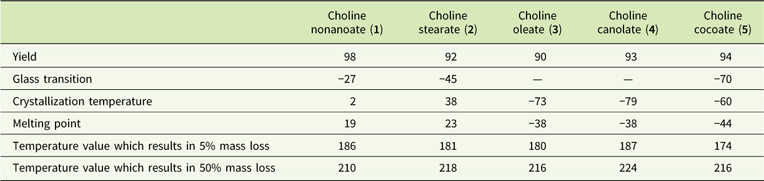
Products were harvested with high yields (Table 1) and were characterized as high-viscosity liquids (choline nonanoate, 1; choline oleate, 3; choline canolate, 4; choline cocoate, 5) or solid (choline stearate, 2) with melting points below 100C. Glass transitions of compounds containing saturated anions (choline nonanoate, 1; choline stearate, 2; choline cocoate, 5) were observed at temperatures from −70 C (choline cocoate, 5) to −27C (choline nonanoate, 1). No glass transitions occurred for ILs 3 and 4. Crystallization and melting temperatures varied from −79 C to 38 C and −44 C to 23 C, respectively. These obtained ILs can be described as thermally stable. No notable influence of the anion structure was observed. Temperatures of decomposition of 5% of the samples were determined to range from 174 to 187 C. The synthesized ILs are classified as room-temperature ionic liquids.
Table 1 Synthetic yield and physical properties of choline carboxylates.
Chemical structures of the synthesized products were confirmed by analysis of 1H and 13C NMR spectra. Protons in the methyl groups of the cation generated a single strong signal in the range of 3.24 to 3.28 ppm. Methylene protons in position α to the quaternary nitrogen generated a signal at 3.51 to 3.61 ppm, while protons in position β occurred as a signal between 3.97 and 4.03 ppm. Signals originating from the anion were identified as peaks at 0.87 to 0.88 ppm for methyl protons and at 1.23 to 1.35 ppm for methylene groups, with two signals at 1.48 to 1.55 ppm and 2.04 to 2.12 ppm for protons in positions that are β and α to the carboxyl group, respectively.
Solubility
Solubility of the synthesized ILs was strongly influenced by their chemical structures (Table 2). All ILs are soluble in chloroform and methanol and express limited solubility in water. No ILs are soluble in hexane, toluene, or ethyl acetate. ILs with a longer alkyl chain in the anion (choline stearate, 2; choline oleate, 3; choline canolate, 4) are less soluble in water than ILs with shorter alkyl substituents (choline nonanoate, 1; choline cocoate, 5).
Table 2 Solubilities of prepared ionic liquids.

a Solubility: +, complete; ±, limited; –, insoluble.
Surface Activity
Aqueous solutions of ILs were investigated for their surface activities (Table 3). The following surface activity parameters were characterized: critical micelle concentration (CMC), surface tension at CMC (γCMC), effectiveness of surface-tension reduction (πCMC), maximum surface excess concentration (Γmax), and surface area occupied by the IL compound (Amin). Anion structure strongly influences the surface activity of the synthesized IL. The highest micelle concentration (CMC) was noted for choline nonanoate (1), which contains the shortest saturated alkyl chain in the anion. Elongating the saturated alkyl substituent results in a lower CMC value (choline stearate, 2). An unsaturated bond (choline oleate, 3) significantly decreases CMC relative to choline nonanoate. Results obtained for anions of natural origin (choline fatty acids from canola [4] and choline fatty acids from coconut oil [5]) confirm this relationship. The CMC for IL 5, which consists of a mixture of short saturated substituents, places it between the results obtained for ILs 1 and 2. For IL 4, test results are consistent with predictions—rapeseed oil contains mainly oleic acid; however, small amounts of different acids, including saturated ones, are also present.
Table 3 Surface parameters of the synthesized ionic liquids.Footnote a
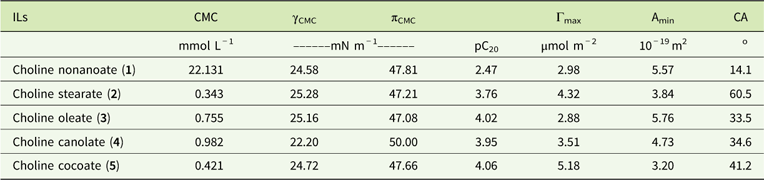
a Abbreviations: γCMC, surface tension at the CMC; πCMC, effectiveness of surface-tension reduction; pC20, the negative logarithm of the surfactant concentration in the bulk phase required to reduce the surface tension of the water by 20 mN/m; Γmax, maximum surface excess concentration; Amin, surface area occupied by the IL compound; CA, contact angle; CMC, critical micelle concentration; ILs, ionic liquids;
Contact angles were determined from analysis of the drop shapes of the ILs on paraffin (Figure 2). Choline nonanoate (1), with a contact angle of 14.1°, produces the lowest contact angle and provides the best wetting of paraffin. Choline stearate, with its longer saturated alkyl chain (2), significantly increases the contact angle to 60.5°. This trend of a longer alkyl chain increasing the contact angle is confirmed by test results for IL 5, which consists of a mixture of saturated anions with moderate alkyl lengths. ILs with unsaturated anions (3, 4) produce comparable contact angles of 33.5° and 34.6°, respectively. All synthesized ILs except IL 2 present better wetting properties than Actirob® (contact angle=42.8°). The high contact angle (60.5°) for choline stearate (2) may be related to the longer saturated alkyl chain in the anion. This phenomenon was observed previously by Pernak and colleagues for herbicidal ILs with long alkyl chains in the cation (Pernak et al. Reference Pernak, Giszter, Biedziak, Niemczak, Olszewski, Marcinkowska and Praczyk2017).
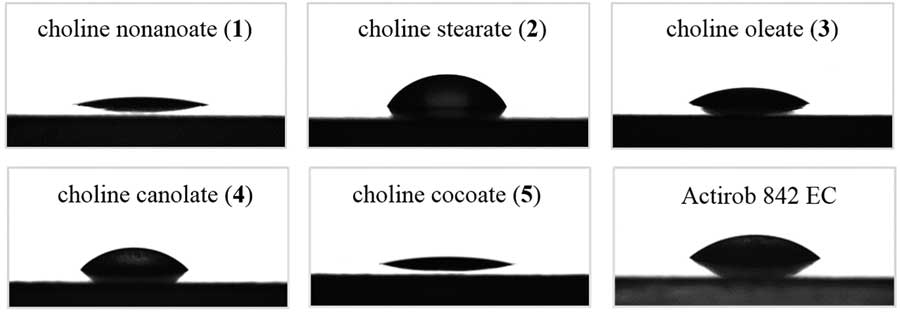
Figure 2 Drop shapes of ionic liquids on a hydrophobic surface (paraffin).
Densities of ILs were measured at temperatures ranging from 20 to 80 C (Figure 3). ILs 2 and 5 are highly viscous, so densities of these two ILs are measured at temperatures between 40 and 80 C. ILs with shorter alkyl chains (1, 5) exhibit the highest densities. The presence of unsaturated bonds in ILs synthesized from glyceryl trioleate and canola oil (3, 4) do not alter compound densities when compared with the IL with a saturated stearate anion (2).
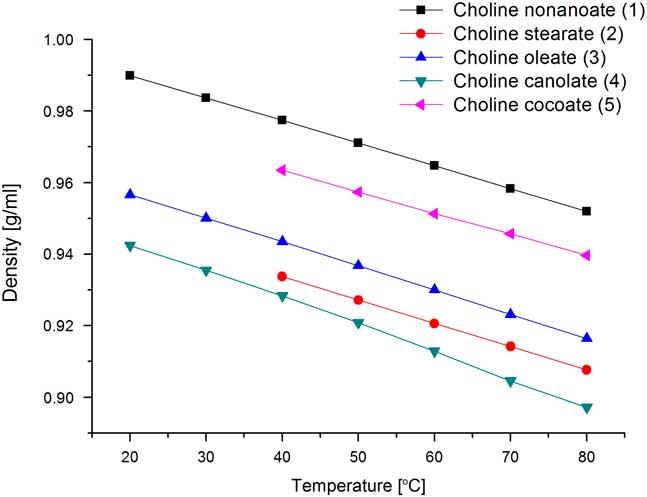
Figure 3 Densities of synthesized ionic liquids.
Deposit of Herbicides—Scanning Electron Microscopy Studies
Leaves of B. napus are covered with a thick layer of well-developed epicuticular wax and are difficult to wet. ILs, when applied as herbicide spray adjuvants, significantly affect residual patterns of spray droplets on B. napus leaves (Figure 4). ILs consisting of choline oleate (3), choline canolate (4), and choline cocoate (5) produce droplet deposits with the largest spread area for all three herbicides and significantly change spray-droplet forms by creating coffee-ring deposit patterns. Ringlike deposits occur when the contact line of the droplet is pinned to the leaf surface. The liquid evaporates from the edge of the droplet and is replenished as liquid from the inner portion of the drop migrates toward the edge (Deegan et al. Reference Deegan, Bakajin, Dupont, Huber, Nagel and Witten2000). Adjuvants that produce a large spread area with patterns that look like coffee rings are most effective in improving herbicidal activity. These test results suggest that ILs 3, 4, and 5 will maximize herbicidal activities of metsulfuron-methyl, iodosulfuron-methyl-sodium, and tribenuron-methyl.
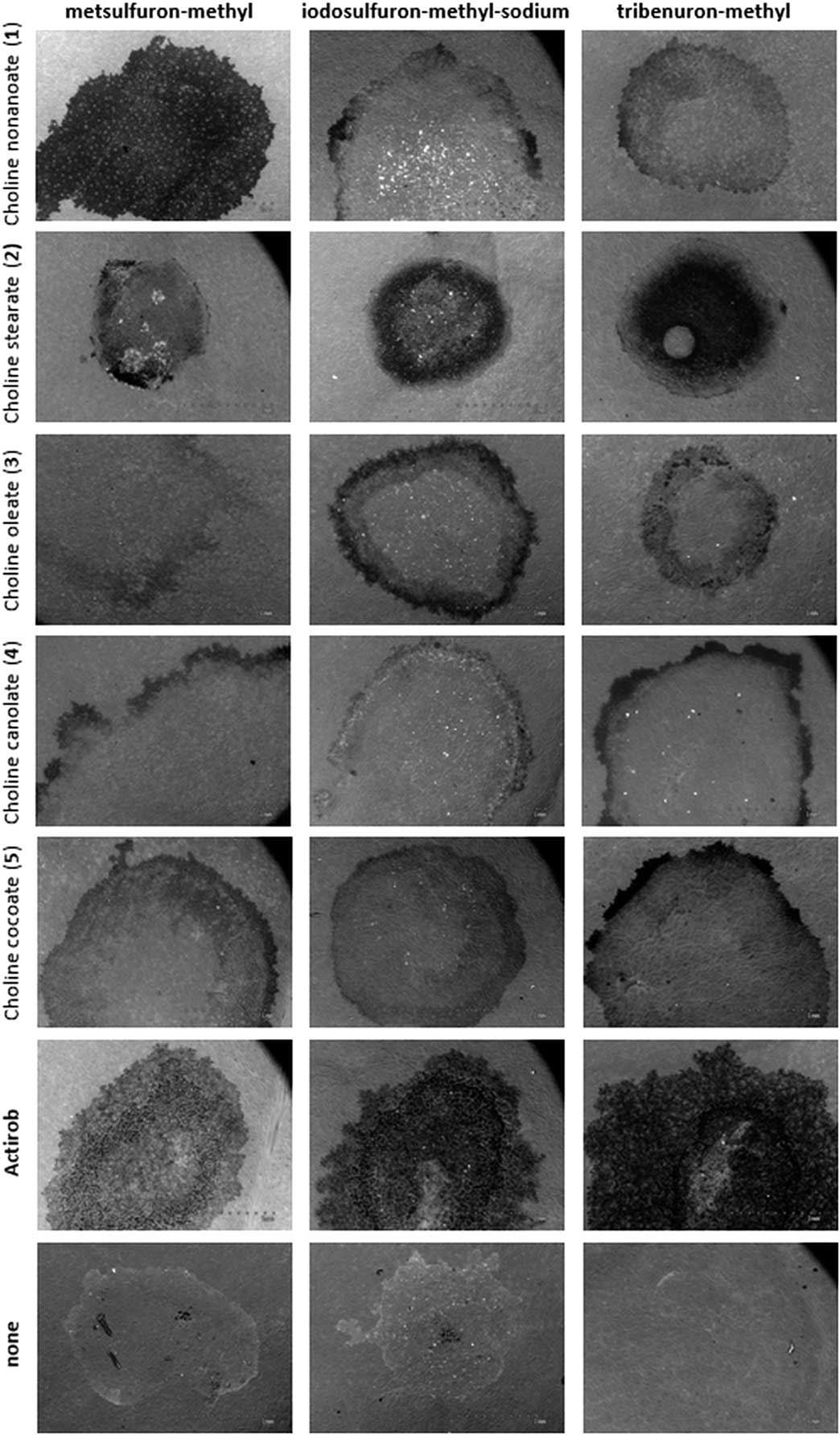
Figure 4 Scanning electron micrographs of herbicide deposits with adjuvants on leaves of oil-seed rape.
Herbicidal Efficacy Trials
Adjuvants and Metsulfuron-methyl
Papaver rhoeas and C. album are most sensitive to metsulfuron-methyl. When averaged across both experiments, metsulfuron-methyl at 4 g ha−1, applied alone, provides 84%, 71%, 48%, and 26% control of P. rhoeas, C. album, B. napus, and C. cyanus, respectively (Figures 5 and 6). The addition of IL adjuvant maintains or improves the herbicidal efficacy of metsulfuron-methyl. Herbicide combinations with all ILs at all three concentrations and with Actirob® significantly improve weed control activity by 18% to 24% on C. album (Table 4). In addition, herbicide combinations with ILs with most adjuvant concentrations and with Actirob® significantly increase weed control activity by 22% to 40% on B. napus (Table 4). Although not statistically significant, bioassay results for C. cyanus and P. rhoeas are consistent with bioassay results for C. album and B. napus. Overall, ILs consisting of choline oleate (3), choline plus fatty acids of canola oil (4), and choline plus fatty acids of coconut oil (5) are the most effective adjuvants. Choline nonanoate (1) and choline stearate (2) are slightly less effective in increasing the herbicidal activity of metsulfuron-methyl. The results show that ILs 3, 4, and 5, at concentrations of 0.2% to 0.4%, are as effective as the commercial adjuvant, Actirob®, at 0.75%, for improving the herbicidal activity of metsulfuron-methyl. Improved herbicidal activities with ILs 3, 4, and 5 are consistent with predictions of improved herbicidal activity based on the large spread areas with patterns that look like coffee rings from the droplet-deposition assay.
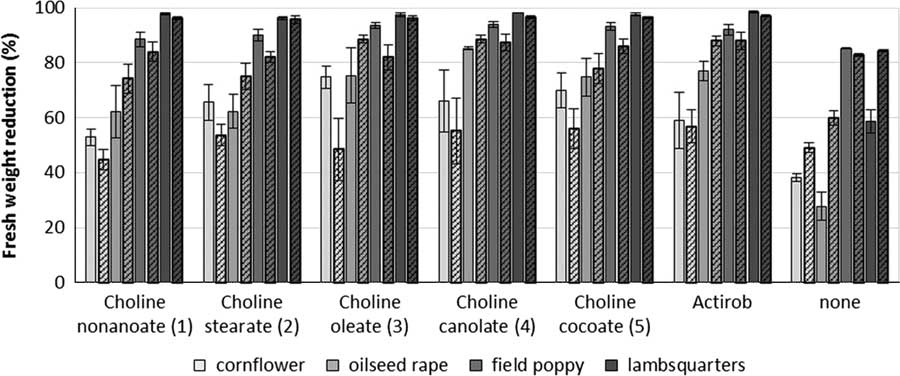
Figure 5 Reduction in fresh weight of plants treated with metsulfuron-methyl at 4 g ai ha−1 plus different bio-ionic liquids at 0.4% concentration or Actirob® at 0.75% concentration at 3 wk after herbicide application. Experiment 1, solid bars; Experiment 2, patterned bars. The error bars represent the SE of the treatment mean.

Figure 6 Chenopodium album response to metsulfuron-methyl, applied alone at 4 g ai ha−1 and with ILs at 0.4% concentration or with Actirob® at 0.75% concentration at 3 wk after herbicide application.
Table 4 Fresh weights of plants treated with metsulfuron-methyl at 4 g ai ha−1 with and without adjuvants at 3 wk after treatment.
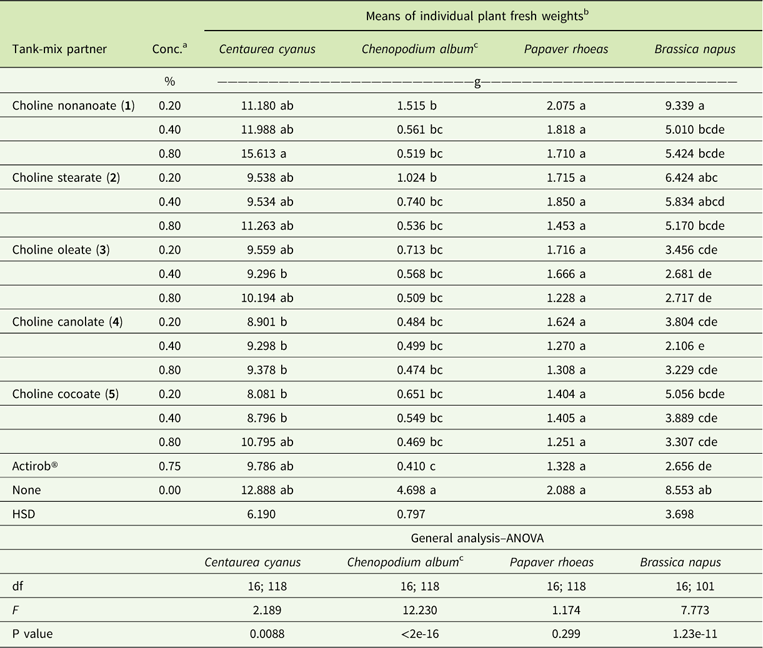
a Abbreviation: Conc., concentration.
b Values followed by the same letter in the same column are not significantly different (P=0.05).
Fresh weights of untreated plants were 17.443, 16.294, 12.914, and 16.294 g for C. cyanus, C. album, P. rhoeas, and B. napus, respectively.
c Data after transformation (λ=−0.061).
Adjuvants and Iodosulfuron-methyl-sodium
Papaver rhoeas and C. album are most sensitive to iodosulfuron-methyl-sodium (Figure 7). When averaged across both experiments, iodosulfuron-methyl-sodium, applied alone at 7.5 g ha−1, provides 88%, 85%, 70%, and 47% control of C. cyanus, C. album, P. rhoeas, and B. napus, respectively. The addition of IL adjuvant maintains or improves the herbicidal efficacy of iodosulfuron-methyl-sodium. When compared with iodosulfuron-methyl-sodium, applied alone without other herbicides, all adjuvants significantly increase the efficacy of the herbicide on C. album and P. rhoeas by 11% to 20% (Table 5). Although not significantly different, bioassay results for C. cyanus, and partially for B. napus, are consistent with those of C. album and P. rhoeas. In most cases, ILs consisting of choline oleate (3), canolate (4), and cocoate (5) are more effective than ILs consisting of choline nonanoate (1) and choline stearate (2). This trend is particularly noticeable for B. napus, the species least susceptible to iodosulfuron-methyl-sodium. ILs 3, 4, and 5, at concentrations from 0.2% to 0.4% v/v, are as effective as Actirob® at 0.75% v/v for improving herbicidal activity of iodosulfuron-methyl-sodium. These bioassay results are consistent with bioassay results for metsulfuron-methyl and with predictions based on the spreading-droplet assay.
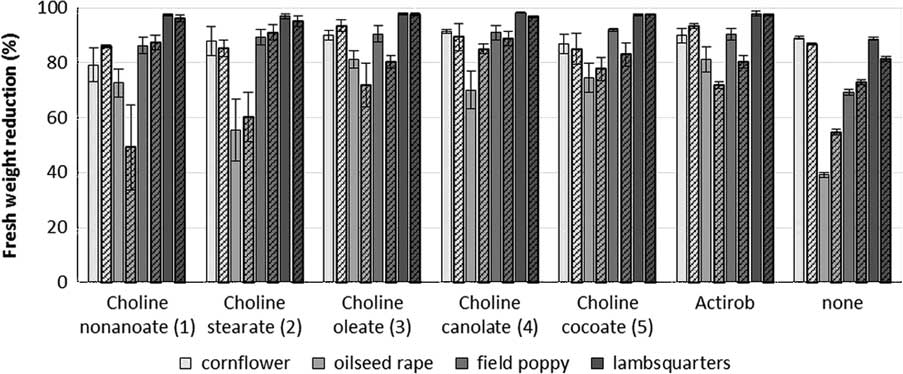
Figure 7 Reduction in fresh weight of plants treated with iodosulfuron-methyl-sodium at 7.5 g ai ha−1 plus different bio-ionic liquids at 0.4% concentration or Actirob® at 0.75% concentration at 3 wk after herbicide application. Experiment 1, solid bars; Experiment 2, patterned bars. The error bars represent the SE of the treatment mean.
Table 5 Fresh weights of plants treated with iodosulfuron-methyl-sodium at 7.5 g ai ha−1 with and without adjuvants at 3 wk after treatment.

a Abbreviation: Conc., concentration.
b Values followed by the same letter in a column indicate no significant difference between treatments. Fresh weights of untreated plants were 26.518, 18.214, 18.282, and 12.948 g for C. cyanus, C. album, P. rhoeas, and B. napus, respectively.
c Data after transformation (λ=−0.182).
d Data after transformation (λ=−0.020).
Adjuvants and Tribenuron-methyl
Chenopodium album, P. rhoeas, and C. cyanus are very sensitive to tribenuron-methyl. When averaged across both experiments, tribenuron-methyl at 15 g ha−1 provides more than 80% control of these weeds (Figure 8). No clear conclusions can be drawn from the C. album, P. rhoeas, or C. cyanus bioassay results, because herbicide use alone exhibits excellent efficacy. Responses of B. napus show the addition of ILs as adjuvants maintains herbicidal efficacy of tribenuron-methyl, and ILs 3, 4, and 5 significantly improve the herbicidal efficacy of tribenuron-methyl to a level similar to that of Actirob® (Table 6). Bioassay results for tribenuron-methyl are consistent with those for metsulfuron-methyl and iodosulfuron-methyl-sodium and with predictions based on the droplet-spread assay.
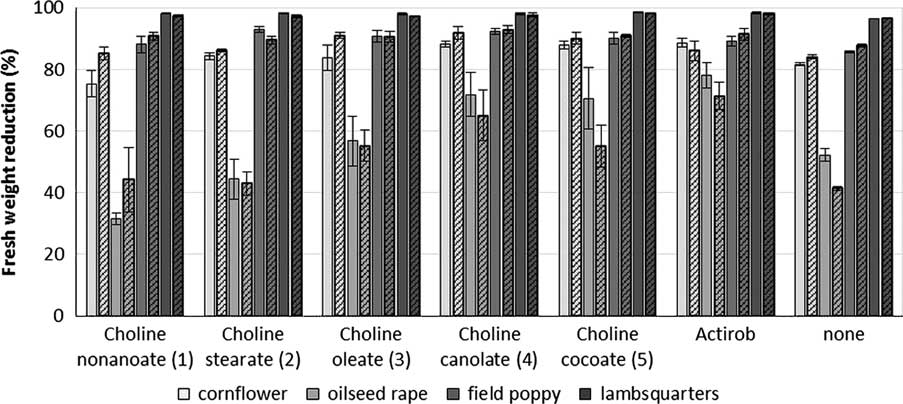
Figure 8 Reduction in fresh weight of plants treated with tribenuron-methyl at 15 g ai ha−1 plus different bio-ionic liquids at 0.4% concentration or Actirob® at 0.75% concentration at 3 wk after herbicide application. Experiment 1, solid bars; Experiment 2, patterned bars. The error bars represent the SE of the treatment mean.
Table 6 Fresh weights of plants treated with tribenuron-methyl at 15 g ai ha−1 with and without adjuvants at 3 wk after application.
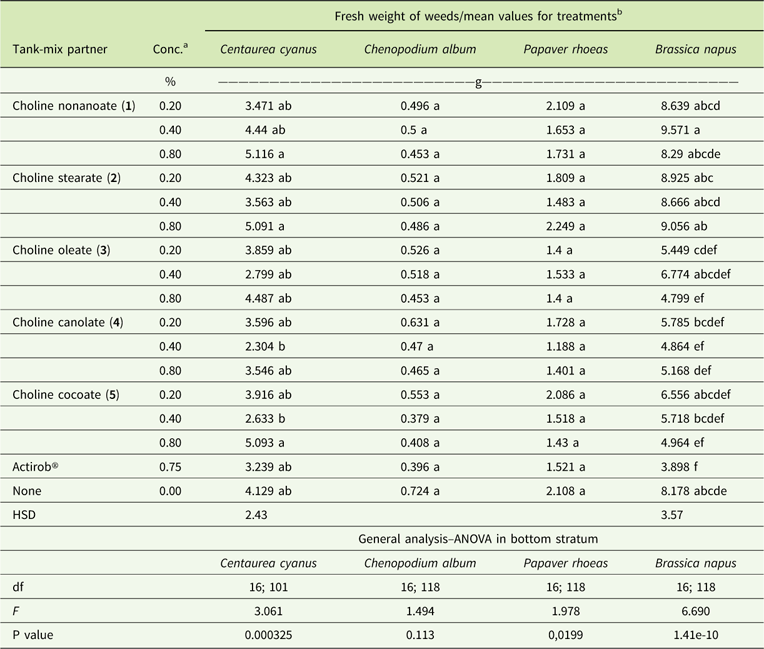
a Abbreviation: Conc., concentration.
b Values followed by the same letter in a column indicate no significant difference between treatments. Fresh weights of untreated plants were 23.888, 21.048, 16.120, and 15.394 g for C. cyanus, C. album, P. rhoeas, and B. napus, respectively.
In summary, specific BILs such as choline oleate, choline plus fatty acid anions from canola oil, and choline plus fatty acid anions from coconut oil are viable candidates for a new class of bio-adjuvants for herbicides. These BILs improve POST herbicidal activities of the sulfonylurea herbicides metsulfuron-methyl, iodosulfuron-methyl-sodium, and tribenuron-methyl to levels similar to that of the methylated seed oil Actirob® 842 EC. Methylated seed soils and nonionic surfactants are currently the preferred adjuvants for sulfonylurea herbicides. BILs are a new type of adjuvant system with the advantage of being synthesized from renewable and sustainable natural products with extremely low levels of toxicity. This adjuvant system might be considered to be “green chemistry” in the herbicide industry. Opportunities to find the correct BIL to meet a particular adjuvant specification are good, because this particular group of compounds contains approximately 1,018 possible cation–anion combinations (Holbrey and Seddon Reference Holbrey and Seddon1999).
Acknowledgements
This work was supported by Ministry of Science and Higher Education, statutory research: Institute of Plant Protection–National Research Institute (number BSOR-11) and Poznan University of Technology (number 03/32/DSPB/0808). No conflicts of interest have been declared.


















