Introduction
Many agricultural weed species are more responsive to fertilizer than major crops (Blackshaw et al. Reference Blackshaw, Brandt, Janzen, Entz, Grant and Derksen2003, Reference Blackshaw, Brandt, Janzen and Entz2004; DiTomaso Reference DiTomaso1995; Little et al. Reference Little, DiTommaso, Westbrook, Ketterings and Mohler2021). Increasing fertilization rates may increase the competitiveness of these weeds, potentially reducing or eliminating the positive effects of fertilization on crops (Blackshaw and Brandt Reference Blackshaw and Brandt2008, Reference Blackshaw and Brandt2009; DiTomaso Reference DiTomaso1995; Little et al. Reference Little, DiTommaso, Westbrook, Ketterings and Mohler2021). For this reason, fertilization methods (rate, source, placement, and timing of nutrients supplied) should ensure that crops have more access to nutrients than weeds (DiTomaso Reference DiTomaso1995; Little et al. Reference Little, DiTommaso, Westbrook, Ketterings and Mohler2021). Precision fertilization approaches may play an important role in cultural weed management (Mohler et al. Reference Mohler, Teasdale and DiTommaso2021), but these approaches may also increase management complexity and do not eliminate nutrient uptake by weeds. Thus, species-specific information about fertility effects on weed–crop competition can help growers develop fertilization and weed management strategies tailored to their operations.
Hairy galinsoga [Galinsoga quadriradiata Cav.; syn.: Galinsoga ciliata (Raf.) S.F. Blake] is an important summer annual agricultural weed species in the Asteraceae, closely related to smallflower galinsoga (Galinsoga parviflora Cav.). It is most likely native to Central America (Canne Reference Canne1977) but is currently found in most temperate and tropical areas of the world (Uva et al. Reference Uva, Neal and DiTomaso1997). It forms associations with arbuscular mycorrhizae (Harley and Harley Reference Harley and Harley1987). Galinsoga quadriradiata has several characteristics that make it a troublesome weed in low-growing crops, especially vegetables, where it can cause yield reductions up to 50% (Warwick and Sweet Reference Warwick and Sweet1983). It is capable of rapid early growth, eventually reaching a height of up to 60 cm with profuse branching (Damalas Reference Damalas2008). In moist conditions, severed or uprooted plants can reestablish (Damalas Reference Damalas2008). Galinsoga quadriradiata can begin flowering as early as 30 to 40 d after emergence (Brainard Reference Brainard2002) and produce up to 1.3 million seeds m−2 in unmanaged fields (Kumar et al. Reference Kumar, Brainard and Bellinder2009). The seeds appear to lack primary dormancy and exhibit low rates of secondary dormancy (De Cauwer et al. Reference De Cauwer, Devos, Claerhout, Bulcke and Reheul2014). Rapid reproduction, coupled with the lack of seed dormancy, gives G. quadriradiata the ability to complete two or more generations in a single growing season in the northeastern United States.
Control options for G. quadriradiata are limited. Some herbicides registered for use in vegetable crops are not very effective against this species (Kumar et al. Reference Kumar, Brainard and Bellinder2009). Mechanical control can also be difficult, because G. quadriradiata may emerge at high densities over a long emergence period, grows rapidly, and can reestablish from severed or uprooted stems (Damalas Reference Damalas2008; De Cauwer et al. Reference De Cauwer, De Cuypere, De Ryck, Delanote, DeWaele, Willekens and Reheul2019). Difficulties with chemical and mechanical management increase the importance of cultural strategies to promote crop competitiveness relative to G. quadriradiata. These strategies should include fertilization practices that adequately fertilize crops without unnecessarily increasing competition from this weed. Such fertilization practices should be informed by knowledge about the nutrient responses of G. quadriradiata.
Increased soil fertility may increase the competitiveness of G. quadriradiata. In one site-year of a field experiment on weed suppression from a rolled cereal rye (Secale cereale L.) cover crop, G. quadriradiata was associated with the highest rate of poultry litter (160 kg N ha−1) according to an indicator species analysis (Ryan et al. Reference Ryan, Curran, Grantham, Hunsberger, Mirsky, Mortensen, Nord and Wilson2011). A greenhouse experiment found that elevated nitrogen increased the competitiveness of G. quadriradiata relative to confamilial species [common sowthistle (Sonchus oleraceus L.) and Heteropappus hispidus (Thunb.) Less.] (Liu et al. Reference Liu, Yang and Zhu2018). Recently, De Cauwer et al. (Reference De Cauwer, Biesemans, De Ryck, Delanote, Dewaele, Willekens, Vanden Nest and Reheul2021) reported that soils with lower levels of plant-available phosphorus had smaller G. quadriradiata seedbanks. They suggested that growers seeking to control this weed should use organic fertility amendments low in readily available phosphorus. Overall, these studies illustrate the capacity of G. quadriradiata to thrive in highly competitive environments, especially when it is not nutrient limited.
The objective of our study was to assess how nitrogen and phosphorus affect biomass and flower production of G. quadriradiata, as well as its competitiveness against lettuce (Lactuca sativa L.). Given that G. quadriradiata has been observed to thrive in highly competitive environments when nutrients are not limiting, we hypothesized that increasing nutrient rates would benefit G. quadriradiata over lettuce.
Materials and Methods
The study consisted of two experiments. The greenhouse experiment, conducted in the fall of 2013, examined how G. quadriradiata growth and flower production responded to nitrogen and phosphorus rates. The field experiment, conducted in the summer of 2014, had the same goal, but also examined how nitrogen and phosphorus rates affected competition between G. quadriradiata and lettuce.
Galinsoga quadriradiata seeds were collected in late summer of 2013 from a garden population in Slaterville Springs, NY, USA (42.40°N, 76.35°W). The lettuce variety was ‘New Red Fire MT0’ (Johnny’s Selected Seeds, Winslow, ME, USA). Sixteen nutrient rate combinations were used. In the “nitrogen series,” five nitrogen rates of 0, 45, 90, 180, or 360 kg N ha−1 were applied in a low- (0 kg P2O5 ha−1) or high- (140 kg P2O5 ha−1) phosphorus environment. In the “phosphorus series,” five phosphorus rates of 0, 35, 70, 140, or 280 kg P2O5 ha−1 were applied in a low- (0 kg N ha−1) or high- (180 kg N ha−1) nitrogen environment. Nitrogen was applied as ammonium nitrate, NH4NO3, and phosphorus was applied as triple superphosphate, Ca(H2PO4)2·H2O. Lime was added to treatments such that calcium addition for all treatments was the same as for the highest rate of triple superphosphate.
Greenhouse Experiment
A randomized block design was used. Pots were arranged in eight blocks, each containing one 15-cm pot for each of the 16 nutrient treatments. The potting medium was a mixture of decompressed peat moss, horticultural vermiculite, and field soil in a 1:1:4 ratio by volume. The field soil was a Mardin channery silt loam (coarse-loamy, mixed, active, mesic Typic Fragiudepts). The field soil would have provided most of the nutrients for seedling establishment. In 2011, this soil had a pH of 4.1, an organic matter content of 4.5%, 323 kg ha−1 of nitrate, and 12 kg ha−1 of elemental phosphorus (Morgan extraction). The field soil was screened through 1.3-cm hardware cloth before use. Each pot was filled with moist potting mix fertilized with nitrogen and phosphorus according to its nutrient treatment. Fifteen G. quadriradiata seeds were distributed on the surface of the pot and covered with a thin layer of dry potting mix from the same nutrient treatment. Finally, each pot was lightly watered to improve seed contact with the potting mix.
The plants were kept in the greenhouse for 49 d at 28/21 C day/night temperatures. Natural light was supplemented with 1,000-W metal-halide lights between 8 AM and 6 PM. Positions of blocks and of plants within blocks were rerandomized weekly. The pots were initially surface watered twice a day. Because pots were observed to dry very fast, 15-cm aluminum pans were placed under the pots at 12 d after planting, and pots were subirrigated for the remainder of the study. Subirrigation kept the pots adequately and uniformly moist and reduced potential loss of nutrients by leaching.
Emergence of G. quadriradiata was not synchronous, which caused high variation in seedling size (data not shown). Some pots did not show emergence by 19 d after planting, so they received seedlings transplanted from pots in the same treatment that had multiple emerged seedlings. Because of seedling mortality, some further transplanting was necessary between 25 and 36 d after planting. Seedlings from pots with multiple living seedlings were transplanted into pots with no living seedlings. The transplants were small relative to the size of the pots and generally established well. Plants were thinned periodically to prevent competition until only 1 plant remained per pot at 36 d after planting. When thinning, we removed the smallest and largest plants in each pot to reduce size variation.
The first open flowers were observed on day 39. As flowers matured, seed heads were collected and saved before seed shed. After 49 d, when all plants had flowered, the total number of flowers on each plant was recorded. Plants were clipped at soil level, and each individual plant was placed in a separate paper bag. Seed heads that had previously been removed from the plants were added to the corresponding bags. The plant materials were dried at 60 C for 7 d. Dried plants were allowed to equilibrate with atmospheric moisture in a lab before they were individually weighed.
Field Experiment
On May 23, 2014, seeds were planted in the greenhouse in 72-cell flats. The seeding rate was 2 seeds per cell for lettuce and 8 seeds per cell for G. quadriradiata. The potting medium was prepared by mixing compressed peat moss, horticultural vermiculite, horticultural perlite, dolomite powder, and AquaGro® 2000G Media Surfactant (Aquatrols, Paulsboro, NJ, USA) at a ratio of 535:180:180:6.25:1, respectively, by volume. Nutrient treatments were the same 16 treatments used in the greenhouse experiment. The potting medium did not contain any field soil that could supply nutrients for seedling establishment, so a commercial fertilizer blend was added (Jack’s Classic All Purpose 20-20-20 with Micronutrients®, JR Peters, Allentown, PA, USA) to all treatments at a rate equivalent to 175 kg ha−1, plus an extra 30 kg ha−1 of ammonium nitrate.
Seedlings were grown in the greenhouse for 17 d under natural light at 30/23 C day/night temperatures. They were watered twice a day, and the positions of flats were randomized weekly. Plants were thinned to 1 individual per cell on days 11 and 12 after planting. In some treatments with low emergence, G. quadriradiata seedlings were transplanted to empty cells instead of being discarded during thinning. On day 18, flats were moved to uncovered cold frames outdoors to harden off seedlings before transplanting them into the field at 24 to 27 d after planting.
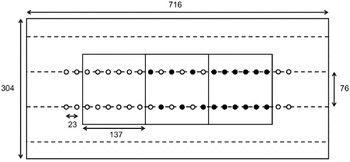
The field selected for the experiment was located at the Musgrave Research Farm, near Aurora, NY, USA (42.73°N, 76.66°W). The soil was a Lima silt loam (fine-loamy, mixed, semiactive, mesic Oxyaquic Hapludalfs) with pH 7.1, 3.4% organic matter, and 3.6 mg kg−1 phosphorus (modified Morgan extraction). Each of the 16 nutrient treatments was replicated five times in a spatially optimized complete block design (van Es et al. Reference van Es, Gomes, Sellmann and van Es2007). Each plot (716 by 304 cm) contained three subplots of either G. quadriradiata, lettuce, or alternating G. quadriradiata and lettuce plants. Each subplot contained 12 plants in two rows of six (Figure 1). The distance between rows was 76 cm, and the distance between plants within rows was 23 cm. The positions of subplots within plots were randomized, and 2 extra lettuce plants were added at the ends of each main plot row to ensure normal competitive conditions at the edges of the plot.

Figure 1. Whole-plot and subplot design for the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. Dimensions are given in centimeters, and subplots were arranged randomly (open circles, lettuce plants; filled circles, Galinsoga quadriradiata plants).
Before transplanting, the field was moldboard plowed and disked to destroy weeds and to incorporate maize (Zea mays L.) residue from the previous growing season. Plot corners were marked with flags, and the appropriate mix of fertilizers was hand broadcast in each plot. A field cultivator was used to incorporate fertilizer into the field, and an empty planter was used to mark rows 76-cm apart. An electrified fence was built around the field to protect plants from animals inhabiting adjacent natural areas.
Seedlings were hand transplanted into the field on June16 to 19, 2014 (24 to 27 d after planting). One or two entire blocks were planted on each day. Plants were given 500 ml of water each immediately after transplanting. The field was largely rainfed, with supplemental watering on June 20 (500 ml per plant) and July 9 to 10 (1,000 ml per plant). The plots were hand cultivated twice, July 1 to 2 and 24 to 25, to prevent competition from naturally establishing weeds. Monthly mean average temperatures were 19.1 C for June and 20.3 C for July (Northeast Regional Climate Center 2022). These values represent 101% and 95%, respectively, of the 30-yr monthly normals (1991 to 2020) of 18.9 C and 21.3 C. Monthly precipitation totals were 7.3 cm for June and 11.7 cm for July (Northeast Regional Climate Center 2022). These values represent 76% and 123%, respectively, of the 30-yr monthly normals of 9.6 cm and 9.6 cm. Therefore, we conclude that temperature conditions were typical for the area and that a relatively wet July may have compensated for a relatively dry June.
The number of flowers produced by a random sample of 3 G. quadriradiata plants in each subplot was recorded July 21 to 23. Plots were harvested August 1 to 5, with replicate blocks harvested in the order in which they were planted. The central 8 of the 12 plants in each subplot were clipped at soil level; the plants at the ends of each subplot were excluded to ensure that all individuals included in the analysis had grown under the same competitive conditions. The G. quadriradiata plants from each subplot were placed together in a large paper bag. The lettuce plants from each subplot were washed in a bucket of water and any lower leaves judged to be below marketable quality were placed in a paper bag. Clean fresh heads of lettuce were weighed and subsequently placed in the corresponding paper bag that contained the lower leaves. All samples were dried at 60 C for 8 d, and dry biomass values were recorded.
Data Analysis
The statistical analysis was performed using R 3.1.0 and 4.1.0 (R Core Team 2021) and SAS v. 9.2 (SAS Institute, Cary, NC, USA). Results were analyzed by examining two nutrient series: the phosphorus series, in which phosphorus was a continuous variable and the nitrogen background environment was either low (0 kg N ha−1) or high (180 kg N ha−1); and the nitrogen series, in which nitrogen was a continuous variable and the phosphorus background environment was either low (0 kg P2O5 ha−1) or high (140 kg P2O5 ha−1).
To analyze biomass data, we first divided subplot sample biomass values in the field experiment by the number of plants harvested to show all data on a per-plant basis. Curvilinearity in biomass responses to nutrient rate was tested with exploratory ANOVAs by examining the significance of a quadratic term. The threshold for further nonlinear analysis was a significance level of P < 0.1. We used this liberal standard for rejection of the null hypothesis of linearity (P < 0.1 rather than P < 0.05) because biological principles dictate that biomass responses to nutrient rate should be asymptotic. When the threshold for curvilinearity was not met (P > 0.1), a second ANOVA was run with only linear terms to test whether biomass was significantly affected by nitrogen or phosphorus rate, the difference between low and high rates of the contrasting nutrient (phosphorus or nitrogen), interactions between the two nutrients, and (in the field experiment) the difference between interspecific and intraspecific competition.
When the threshold for curvilinearity was met, nonlinear regression analysis was performed. We fit the data to an alternative to the Mitscherlich equation (Gardner et al. Reference Gardner, Pearce and Mitchell1985) that is more likely to produce convergence during nonlinear analysis:
where Y is the response variable, a is a fitted parameter representing the asymptotic value of Y, b is a fitted parameter representing the difference between a and the value of Y with no added nutrient, c is a fitted parameter controlling the rate of change in the response variable with increasing nutrient rate, and R is the nutrient rate (in kg ha−1) (Little et al. Reference Little, Mohler, Ketterings and DiTommaso2015). The function nls (package stats) was used to build these models. The function pairComp (package aomisc) was used to perform pairwise comparisons at α = 0.05 with a Holm correction (Onofri Reference Onofri2021).
We analyzed the response of flower production to nutrient rates in the same manner as described for the biomass data. In addition, we evaluated the relationship between dry biomass and flower production in G. quadriradiata. Preliminary analyses showed that the threshold for curvilinearity was not met in either experiment (P > 0.05), so the relationship between dry biomass and flower production in each experiment was evaluated with a linear model and ANOVA.
Results and Discussion
Galinsoga quadriradiata Response to Nutrient Addition
In the greenhouse experiment, phosphorus had a positive linear effect on the dry biomass of G. quadriradiata (P < 0.001; Figure 2). However, no significant effect of nitrogen was observed in either the nitrogen series (data not shown) or the phosphorus series (Figure 2). Flower production was proportional to plant dry biomass (P < 0.001; Figure 3). The fitted model for the relationship between flower production per plant (count) and dry biomass (g) was y = −10.6 + 24.5x (R2 = 0.73).
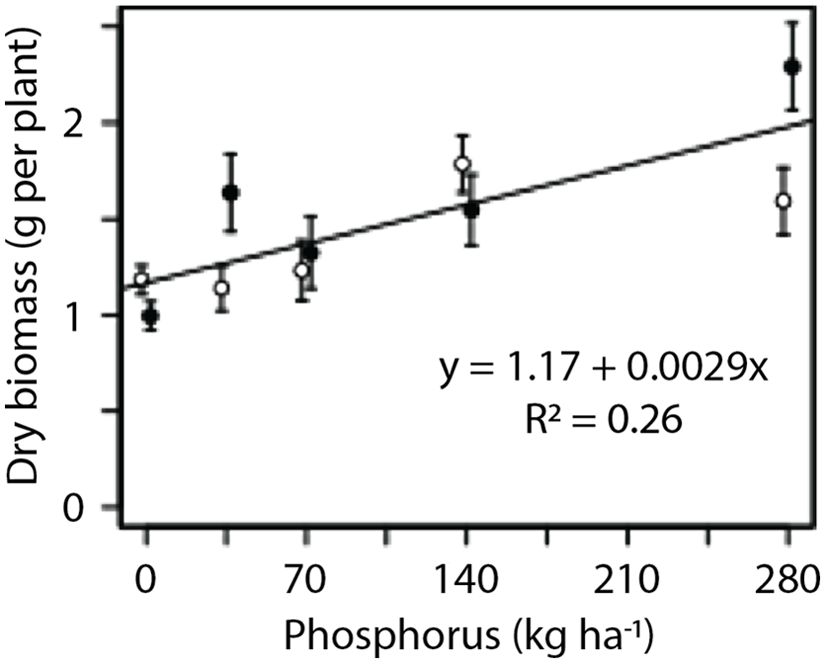
Figure 2. Response of Galinsoga quadriradiata dry biomass to phosphorus rate in the greenhouse experiment conducted in 2013 at Cornell University, Ithaca, NY, USA. Phosphorus was added in low-nitrogen (open circles) or high-nitrogen (filled circles) environments. Nitrogen level did not affect dry biomass or response to phosphorus, so a single model was fit to the data.

Figure 3. Relationship between Galinsoga quadriradiata dry biomass and flower production in the greenhouse experiment conducted in 2013 at Cornell University, Ithaca, NY, USA. Each observation represents one plant (both nitrogen and phosphorus series).
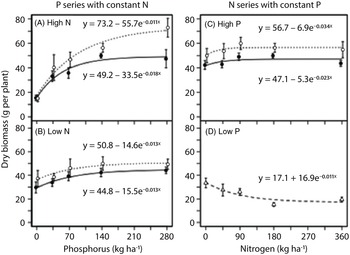
In the field experiment, the effect of nutrient addition on dry biomass of G. quadriradiata was nonlinear (Figure 4; Table 1). Dry biomass of G. quadriradiata responded more strongly to phosphorus than to nitrogen. At high nitrogen, phosphorus rate had a strong positive effect on dry biomass of G. quadriradiata (Figure 4A). The increase in dry biomass from no phosphorus addition to the highest phosphorus rate was greater than 300% in the biculture and greater than 200% in the monoculture. At low nitrogen, the increase in dry biomass with increasing phosphorus was more modest, approximately 30% to 50% (Figure 4B). At high phosphorus, dry biomass reached its maximum at a relatively low nitrogen rate (∼90 kg N ha−1), both in biculture and monoculture (Figure 4C). At low phosphorus, increasing nitrogen rates did not increase the dry biomass of G. quadriradiata (Figure 4D); in fact, parameter b (controlling the direction and magnitude of the effect of nutrient addition on biomass) was negative in the fitted model (Table 1). In general, the dry biomass of G. quadriradiata was similar between the biculture (competition with lettuce) and monoculture (self-competition) treatments. However, in the phosphorus series at high nitrogen, the asymptotic biomass (parameter a) was higher in the biculture than the monoculture (Figure 4A; Table 1).

Figure 4. Response of Galinsoga quadriradiata dry biomass to nitrogen and phosphorus rates in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. Data are shown for the phosphorous series in (A) a high-nitrogen environment and (B) a low-nitrogen environment and for the nitrogen series in (C) a high-phosphorus environment and (D) a low-phosphorus environment. In the low-phosphorus environment for the nitrogen series, biculture and monoculture treatments were aggregated because they did not differ [Open circles and dotted curves, biculture (G. quadriradiata and lettuce); filled circles and solid curves, monoculture; open triangles and dashed curve, aggregated biculture and monoculture].
Table 1. Fitted parameters of regression curves for the response of Galinsoga quadriradiata dry biomass to nutrient rates and competition with lettuce in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. a

a The model has the form Y = a – be −cR , where Y is dry biomass (in g plant−1), a is a fitted parameter representing the asymptotic dry biomass, b is a fitted parameter representing the difference between a and the dry biomass produced without added nutrient, c is a fitted parameter controlling the rate of change in dry biomass with increasing nutrient rate, and R is the added nutrient rate (in kg ha −1). Parameter values within a series followed by the same letter are not significantly different at α = 0.05.
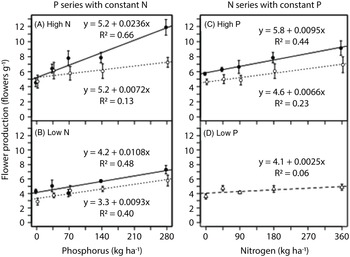
As in the greenhouse experiment, flower production was proportional to plant dry biomass in the field experiment (Figure 5). Rate of both phosphorus and nitrogen generally had positive linear effects on flowers produced per unit mass (Figure 6), except that increasing nitrogen rate had a negligible effect when no phosphorus was applied (Figure 6D). Plants tended to produce more flowers per unit mass when grown in monoculture rather than biculture, especially in the phosphorus series at high nitrogen (Figure 6), in which phosphorus addition (280 vs. 0 kg ha−1) increased flower production per unit mass by 130% in monoculture and 40% in biculture (Figure 6A).

Figure 5. Relationship between mean dry biomass and mean flower production per Galinsoga quadriradiata plant in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. Each observation represents one subplot.

Figure 6. Response of flower production per unit mass of Galinsoga quadriradiata to nitrogen and phosphorus rates in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. Data are shown for the phosphorous series in (A) a high-nitrogen environment and (B) a low-nitrogen environment and for the nitrogen series in (C) a high-phosphorus environment and (D) a low-phosphorus environment. In the low-phosphorus environment for the nitrogen series, biculture and monoculture treatments were aggregated because they did not differ [open circles and dotted lines, biculture (G. quadriradiata and lettuce); filled circles and solid lines, monoculture; open triangles and dashed line, aggregated biculture and monoculture].
To summarize, both greenhouse and field experiments demonstrated positive effects of phosphorus addition on G. quadriradiata biomass. In the field experiment, the positive effect of phosphorus was larger in the presence of high nitrogen. Galinsoga quadriradiata plants with high biomass tended to have more flowers than those with lower biomass. Flower production per unit mass generally increased with nutrient addition. Thus, increased fertility is likely to increase G. quadriradiata seed production (not measured in this study) both by increasing biomass production and by increasing flower production per unit mass. Fertility conditions might also affect other stages of the G. quadriradiata life cycle, such as germination and emergence rates.
Our results are consistent with previous reports that G. quadriradiata is highly responsive to nutrient addition. The finding that phosphorus addition increased biomass and flower production corroborates a report that phosphorus has positive effects on G. quadriradiata growth and flowering (Ivany 1971, cited by Warwick and Sweet Reference Warwick and Sweet1983). Galinsoga quadriradiata has been associated with a high rate of poultry litter (Ryan et al. Reference Ryan, Curran, Grantham, Hunsberger, Mirsky, Mortensen, Nord and Wilson2011). High rates of poultry manure are often associated with excessive phosphorus levels in agricultural fields (Magdoff and van Es Reference Magdoff and van Es2021). Soils with low levels of plant-available phosphorus may have smaller G. quadriradiata seedbanks (De Cauwer et al. Reference De Cauwer, Biesemans, De Ryck, Delanote, Dewaele, Willekens, Vanden Nest and Reheul2021). In our field experiment, biomass and flower production were highest when both nitrogen and phosphorus rates were high. Similarly, previous research has demonstrated that nitrogen addition increases biomass and seed production in G. quadriradiata (Liu et al. Reference Liu, Yang and Zhu2018; Rai and Tripathi Reference Rai and Tripathi1986). Thus, our findings confirm that G. quadriradiata thrives in fertile soils and highlight the strong influence of phosphorus availability on its performance.
Lettuce Response to Nutrient Addition
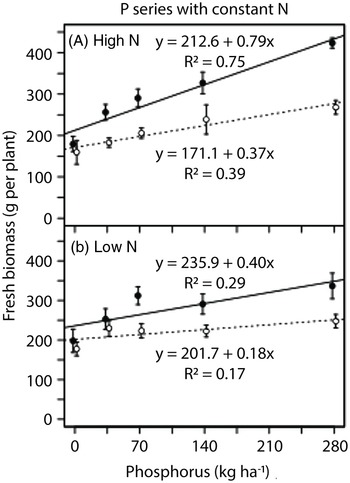
Fresh biomass of lettuce increased linearly in response to phosphorus addition (Figure 7). In the phosphorus series at the high nitrogen rate, lettuce fresh biomass doubled in monoculture and increased by 60% in biculture at the highest phosphorus rate compared with no phosphorus addition (280 vs. 0 kg P2O5 ha−1; Figure 7A). The rate of increase in fresh lettuce biomass in response to phosphorus addition was higher in the high-nitrogen monoculture compared with the high-nitrogen biculture, low-nitrogen monoculture, or low-nitrogen biculture (Table 2). In the nitrogen series, lettuce fresh biomass was not strongly responsive to nitrogen addition (Table 2). When grown without nitrogen addition, lettuce fresh biomass was highest in the high-phosphorus monoculture (Table 2).

Figure 7. Response of lettuce fresh biomass to nitrogen and phosphorus rates in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. Data are shown for the phosphorous series in (A) a high-nitrogen environment and (B) a low-nitrogen environment [open circles and dotted lines, biculture (Galinsoga quadriradiata and lettuce); filled circles and solid lines, monoculture].
Table 2. Response of lettuce fresh biomass to nutrient rates and competition with Galinsoga quadriradiata in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. a

a Data were fit to a linear model, Y = a + bR, where Y is fresh biomass, a is fresh biomass without added nutrient, and b controls the rate of change in fresh biomass with increasing nutrient rates. Parameter values within a series followed by the same letter are not significantly different at α = 0.05.
In contrast with fresh biomass, lettuce dry biomass showed no significant relationship with phosphorus rate in the phosphorus series or nitrogen rate in the nitrogen series (Table 3). Lettuce dry biomass was slightly higher in monoculture than in biculture when averaged across the phosphorous series in the low-nitrogen environment (21 vs. 16 g plant−1; P = 0.05) or when averaged across the nitrogen series in the high-phosphorus environment (22 vs. 17 g plant−1; P = 0.04). Lettuce dry biomass was higher at high phosphorus than low phosphorus when averaged across the nitrogen series in monoculture (22 vs. 17 g plant−1; P = 0.02). Nutrient background environment did not affect lettuce dry biomass in the nitrogen series biculture or the phosphorus series.
Table 3. Response of mean lettuce dry biomass to nutrient rates and competition with Galinsoga quadriradiata in the field experiment conducted in 2014 at Cornell University Musgrave Research Farm, Aurora, NY, USA. a
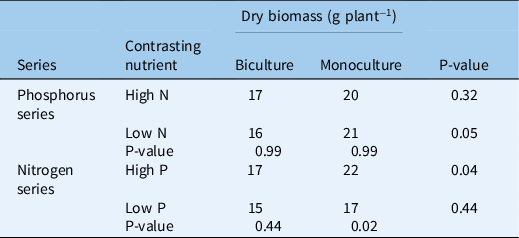
a Because the regressions for the nitrogen and phosphorus addition series were not significant, each value in the table is the mean of the addition series, e.g., the value labeled “Biculture High N” is the mean of the five phosphorus rate treatments in biculture in the high-nitrogen environment. P-values in the rightmost column indicate whether biculture and monoculture treatments differ within nutrient series and contrasting nutrient environment. P-values below nutrient series indicate whether contrasting nutrient environments differ within nutrient series and biculture or monoculture treatment.
The fact that phosphorus addition generally did not affect lettuce dry biomass was surprising, because the soil test indicated low available phosphorus in the unamended soil. The highest addition rates of nitrogen and phosphorus tested in our experiment exceeded the recommended rates for lettuce production (Cornell Cooperative Extension 2014). It is possible that lettuce dry biomass was limited largely by some factor other than phosphorus or nitrogen. Discrepancies between fresh and dry biomass sometimes occur because fresh lettuce has a very high water content (greater than 90%; Sakamoto and Suzuki Reference Sakamoto and Suzuki2015). In our study, high-phosphorus treatments may have produced large, expanded leaf cells containing a large amount of water, while low-phosphorus treatments may have produced slightly smaller cells that had similar dry weights but contained less water. As lettuce is typically sold by fresh weight, phosphorus addition would have increased value in this case.
Implications for Weed–Crop Competition
Comparisons between monoculture and biculture treatments provide insight into the relative competitiveness of G. quadriradiata and lettuce. Dry biomass of G. quadriradiata was usually similar between monoculture and biculture treatments. However, in the phosphorus series in the high-nitrogen environment, G. quadriradiata reached a higher maximum biomass when grown in competition with lettuce than when grown in monoculture. In the phosphorus series at the high phosphorus rates in the high-nitrogen environment, flower production per unit of biomass was higher in the monoculture treatment, indicating that vegetative biomass is not a perfect proxy for reproductive capacity. Nonetheless, our results suggest that high-nutrient conditions promote G. quadriradiata competitiveness against lettuce. Consistent with this interpretation, lettuce biomass (dry or fresh) was sometimes higher in monoculture treatments relative to biculture treatments. For example, lettuce fresh biomass increased at a higher rate with increasing phosphorus in the monoculture treatment, relative to the biculture treatment, in the high-nitrogen environment.
In weed species with strong growth responses to nutrients, nutrient addition may increase competitiveness relative to crop plants (Little et al. Reference Little, DiTommaso, Westbrook, Ketterings and Mohler2021). For example, Blackshaw and Brandt (Reference Blackshaw and Brandt2009) found that the competitiveness of round-leaved mallow (Malva pusilla Sm.) against wheat (Triticum aestivum L.) increased with increasing phosphorus rates. This finding was consistent with an earlier report that M. pusilla exhibits a strong growth response to phosphorus (Blackshaw et al. Reference Blackshaw, Brandt, Janzen and Entz2004). In contrast, the competitive ability of less phosphorus-responsive weed species (kochia [Bassia scoparia (L.) A.J. Scott] and Persian darnel [Lolium persicum Boiss. & Hohen.]) decreased with increasing phosphorous rates (Blackshaw and Brandt Reference Blackshaw and Brandt2009). In some cases, weeds that are strong competitors for nutrients may be highly damaging to crops when nutrients are limited. For example, Santos et al. (Reference Santos, Dusky, Stall, Bewick and Shilling2004a) reported that combined shoot and root interference from common purslane (Portulaca oleracea L.) reduced lettuce shoot dry weight by 84% at a phosphorus rate of 0 g L−1 soil and 71% at 0.8 g L−1 soil. Shrefler et al. (Reference Shrefler, Shilling, Dusky and Brecke1994b) found that phosphorus addition increased the competitiveness of lettuce relative to spiny amaranth (Amaranthus spinosus L.), although this weed remained more competitive than lettuce at all fertility levels. Thus, our finding that phosphorus addition may exacerbate the competitive effect of G. quadriradiata on lettuce is plausible; however, this finding is not necessarily generalizable to other competitive agricultural weed species.
From a management perspective, our results underscore the importance of preventing plant-available phosphorus from reaching excessive levels. Unlike nitrogen, phosphorus is not very mobile in the soil, so it is possible to build a surplus over time in most soil types (Magdoff and van Es Reference Magdoff and van Es2021). Fields that regularly receive organic amendments, such as manure and compost, are especially at risk of phosphorus surpluses, because such amendments often oversupply phosphorus when they are used to fulfill crop nitrogen needs (Magdoff and van Es Reference Magdoff and van Es2021). Excessive phosphorus buildup may lead to multiple issues, including environmental pollution, toxicity to crops, and increased weed–crop competition. To prevent excessive phosphorus buildup and heavy infestations of weeds such as G. quadriradiata, growers can regularly test their soil, calculate crop nutrient requirements each growing season, and apply fertilizer only within the crop rows. For example, banded phosphorus applications can reduce the negative effects of weeds on lettuce yield, relative to broadcast applications (Santos et al. Reference Santos, Dusky, Stall and Gilreath2004b; Shrefler et al. Reference Shrefler, Dusky, Shilling, Brecke and Sanchez1994a). Precision approaches to fertility management are likely to reduce G. quadriradiata performance and help minimize lettuce yield losses.
Acknowledgments
We dedicate this work to Charles L. Mohler. We would like to especially thank Xe García for performing the research and writing an early draft of the manuscript. We thank current and former members of the Weed Ecology and Management Laboratory: Scott Morris, Javaid Iqbal, and undergraduate assistants Kendra Ellis, Elizabeth Kennedy, Kathleen Bitter, Christina Hall, and Melissa Harbert. Other individuals who provided invaluable support include Paul Stachowski and the rest of the staff at the Cornell University Musgrave Research Farm. This project was funded by the Dextra Undergraduate Research Endowment Fund, the CALS Alumni Association Academic Enrichment Program, and Cornell Experiment Station Multi-State Hatch Project NE-1047 (project no. NYC-125800). No conflicts of interest have been declared.