Bronchopulmonary dysplasia (BPD) is a chronic pulmonary disease of multifactorial origin that mainly affects the most immature newborns. Risk factors for this disease were first reported by Northway et al.(Reference Northway, Rosan and Porter1) and remain valid. The increased survival of very low birth weight (VLBW) and extremely low birth weight infants has changed the framework of BPD, from its traditional association with O2 therapy to the consideration of a new condition, which some authors(Reference Coalson2,Reference Yoder, Coalson and Winter3) have termed ‘new BPD’, more closely related to lung immaturity in extreme preterm infants, impaired alveolarisation and fibroproliferation. In parallel to these, however, other factors related to its pathogenesis have recently been identified, such as inflammation and/or infection, pulmonary oedema due to the excessive administration of fluids and/or persistent ductus arteriosus (PDA), increased resistance in the airways, immaturity of antioxidant systems in the newborn, prenatal inflammation, adrenal insufficiency and, of course, genetic predisposition(Reference Yoder, Coalson and Winter3,Reference Kunzmann, Speer and Jobe4) . Nutrition is an important factor in the prenatal formation and growth of the lung and also influences developmental programming through epigenetic changes(Reference Wiedmeier, Joss-Moore and Lane5). The influence of nutrition on lung development continues during postnatal life, especially in early infancy(Reference Joss-Moore, Lane and Albertine6). The inadequate management of nutrition during critical periods of development such as pregnancy and the first days of life, especially in VLBW and extremely low birth weight infants, can alter the normal development of the lungs and contribute to the pathogenesis of BPD(Reference Arigliani, Spinelli and Liguoro7). The nutritional intake during the first week of life is also crucial to the development and maturing of the central nervous system(Reference Stephens, Walden and Gargus8) and other structures.
The association between energy malnutrition and co-morbidities in VLBW infants has been addressed in several previous studies(Reference Stephens, Walden and Gargus8–Reference Uberos, Lardon-Fernandez and Machado-Casas10). In this respect, moreover, it has been reported that critically ill newborns receive less nutrition during the first weeks of life, which provokes a significant delay in establishing complete enteral nutrition (EN) and increases the prevalence of co-morbidities, such as PDA and BPD(Reference Colacci, Murthy and DeRegnier11–Reference Oh13).
It is generally accepted that the supply of nutrients to VLBW infants should be established shortly after delivery, seeking to achieve a growth pattern of the premature newborn similar to that of the fetus before birth. The rapid implementation of EN or, when this is not possible, parenteral nutrition (PN) from the first hours of life is currently recommended by most neonatal care units(Reference Bonsante, Iacobelli and Latorre14,Reference Fischer, Maucort-Boulch and Essomo Megnier-Mbo15) . Nevertheless, and although the theory is clear, the reality is that nutritional intake as obtained by the fetus is rarely achieved. In practice, the situation often encountered is that during the first days of life, the nutritional intake obtained by the newborn is restricted to a greater or lesser extent and with diverse consequences. Among the factors that lead to low-postnatal energy intake, in our opinion and that of other authors(Reference Ziegler16), it is the view that nutrition at the beginning of postnatal life, whether enteral or parenteral, is dangerous, especially in VLBW infants. Consequently, nutrients are introduced only slowly and with great trepidation. As a result, the vast majority of VLBW infants accumulate some degree of postnatal growth restriction during the first weeks of life(Reference Ziegler16).
The malnutrition interferes with pulmonary defences against hyperoxia, volutrauma and infection, all of which are harmful to lung repair, development and maturing(Reference Biniwale and Ehrenkranz17). In addition, premature newborns with BPD usually present growth retardation and cannot normalise respiratory functions(Reference Biniwale and Ehrenkranz17). It has been reported that newborns with BPD present 30–67 % growth delay during the period immediately following hospital discharge, with altered body composition and a deficit in free and total fat, which may persist beyond infancy(Reference Ehrenkranz, Das and Wrage9,Reference Huysman, De Ridder and de Bruin18) .
The aim of the present study is to determine, from the results obtained, the existence of association between BPD in VLBW and energy intake during the first week of life.
Subjects and methods
This retrospective cohort study was conducted on neonates born at the San Cecilio Clinical Hospital (Granada, Spain) between January 2008 and December 2018. The study protocol was approved by the Ethics Committee of the Hospital, and all current regulations regarding data confidentiality were respected.
Inclusion and exclusion criteria
All newborns weighing <1500 g admitted to the neonatal intensive care unit during the study period were included in our analysis. Subsequently, each child was followed in the Pulmonology, Neonatology and Neurology consultations. Infants who died in the first 28 d of life and those transferred to other hospitals were excluded from the analysis, as in these cases it was not possible to establish the degree of BPD. Also excluded were newborns for whom data were not available on total nutritional intake during the first week of life. Fig. 1 shows the flow diagram representing the process applied for patient recruitment and inclusion.
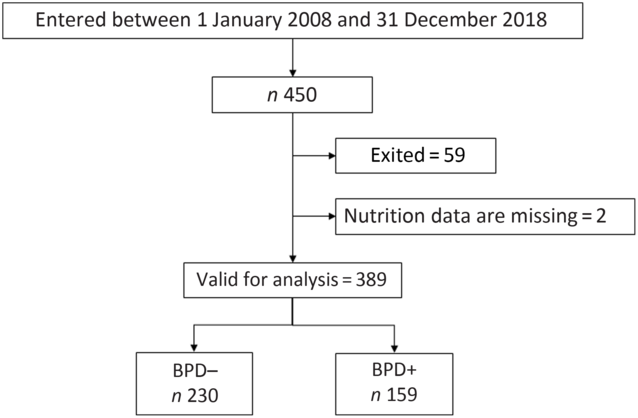
Fig. 1. Flow diagram for the very low birth weight newborns included in the study. BPD, bronchopulmonary dysplasia; BPD–, without BPD; BPD+, with BPD.
Anthropometry
Table 1 shows the weights and z-scores obtained at birth and during the first week of life. Fenton tables were used to calculate the z-scores(Reference Fenton and Sauve19). Intra-uterine growth restriction is defined by decrease in the rate of weight increase, reflected as a weight below the 10th percentile for gestational age(Reference Levine, Grunau and McAuliffe20).
Table 1. Pregnancy and neonatal characteristics
(Numbers and percentages; medians and interquartile ranges (IQR))
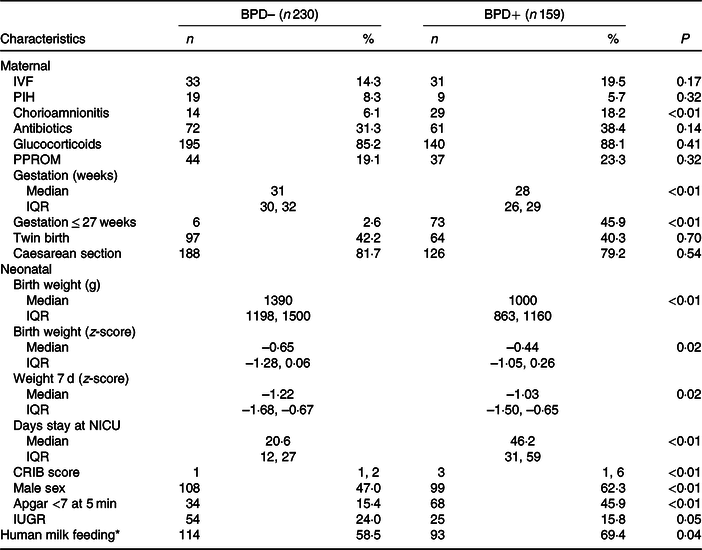
BPD, bronchopulmonary dysplasia; BPD–, without BPD; BPD+, with BPD; IVF, in vitro fertilisation; PIH, pregnancy-induced hypertension; PPROM, preterm pre-labour rupture of membranes; NICU, neonatal intensive care unit; CRIB, clinical risk index for babies; IUGR, intra-uterine growth restriction.
* Supplemented by less than 25 % of the weekly volume with premature formula milk.
Nutritional management
The nutritional strategy applied and the intake of liquids in the neonatal unit were in accordance with its own standard protocol and with the recommendations of the Nutrition and Metabolism Group of the Spanish Neonatology Society(Reference Narbona, Uberos and Armada21,Reference Uberos, Narbona and Gormaz22) . In all cases, 1·2 µm filters are used for PN (Pall, Medical). Two changes were made to this nutritional practice during the study period. In April 2011, the first-day PN (early PN) began to be applied with an amino acid solution (2 g/kg) and with glucose at 5 mg/kg per min. Previously, amino acid had not been supplied during the first hours of life. This change, bringing our action protocols in line with international recommendations, increased the newborns’ energy intake in the first week of life and, secondarily, enabled us to assess the energy repercussions more effectively. The second modification concerned the lipid emulsion used. In July 2016, SMOFlipid (Fresenius Kabi) was introduced, replacing Intralipid (Fresenius Kabi) as an emulsion commonly used in PN. The repercussions of this change with respect to energy intake are taken into account in our analysis of the results.
The normal procedure during the first days of life is to complement EN with PN when complete EN cannot be established. The daily requirements of liquids, proteins and lipids are calculated daily. In all cases, the aim is that during the first week of life the minimum nutritional requirements to ensure growth should be met, according to the standard recommendations(Reference Thureen23). For the purposes of the present study, the inputs of liquids, energy, proteins, carbohydrates and lipids during the first week of life were recorded. We propose that an energy intake below the 25th percentile represents in our sample a situation of energy restriction, and so this was chosen as the cut-off point for BPD risk. This would represent approximately 60 % of the recommended energy intake during the first week.
Morbidity
According to the thresholds proposed by National Institutes of Health consensus definition(Reference Ehrenkranz, Walsh and Vohr24) and by Jobe & Bancalari(Reference Jobe and Bancalari25), BPD is defined as a need for supplemental O2 >21 % at 28 d of life and/or a need for supplemental O2 >21 % or for positive airway pressure at 36 weeks’ corrected gestational age.
A diagnosis of clinical sepsis is made when a NOSEP-1 score (nosocomial sepsis score) >8 is recorded. On this scale, the presence of C-reactive protein > 0·014 g/l is assigned five points; that of neutrophils >50 %, three points; thrombocytopaenia <150 × 109/l, five points and fever >38·2°C, five points(Reference Mahieu, De Muynck and De Dooy26). The clinical risk index for babies (CRIB II score) for each newborn was performed using the following variables: sex, gestational age (in weeks), birth weight (in g) and excess base. The total CRIB II score (range 0 to 27) was calculated(Reference Romagnoli, Giannantonio and De Carolis27).
The diagnosis and staging of retinopathy of prematurity are based on retinal examination before discharge from the neonatal unit(28,Reference Lad, Hernandez-Boussard and Morton29) .
PDA was diagnosed by Doppler ultrasound and treated when clinical repercussions were observed or when the diameter was > 2 mm.
The diagnosis of intraventricular haemorrhage (IVH) is based on Papile’s classification(Reference Papile, Burstein and Burstein30). All neonates receive a transfontanellar ultrasound examination on the third day of life and every week thereafter.
For the diagnosis of necrotising enterocolitis, patients are classified according to Bell’s criteria(Reference Bell31). Cases classified as spontaneous intestinal perforations were excluded from this diagnosis.
Sample size
Previous studies have shown that energy intake can be associated with the risk of developing BPD(Reference Uberos, Lardon-Fernandez and Machado-Casas10). The average prevalence of BPD in our hospital during the last 10 years was approximately 15 % (R0 = 15 %). After reviewing our data and the literature in this respect, we suggest that a nutritional energy deficit in the first week of life can double the risk of developing BPD (R1 = 30 %). To determine the sample size needed for the present study, a two-tailed test with a significance level of 5 % was performed with a power of 90 %. Using the arcsine approximation to calculate the sample size, the results obtained showed that the study cohort should include at least 159 VLBW infants in each group.
Statistical analysis
The descriptive data are summarised using medians (p50) and interquartile range (p25–p75) for the continuous values and frequency distribution for the categorical ones. Univariate comparisons of the continuous variables were performed using the Mann–Whitney U test and by the χ 2 test for the categorical ones. The risk assessment of BPD, retinopathy of prematurity, necrotising enterocolitis, intraventricular haemorrhage and late sepsis was obtained first by means of univariate regression analysis and later by multivariate logistic regression analysis. The weight of the newborn was strongly associated with gestational age and so this variable was not taken into account, in order to avoid over-adjustment phenomena that might bias the associations obtained in the regression analysis. It was decided not to adjust for the CRIB index because it was calculated from the variables, gestational age, weight, male sex, among others, that have already been taken into account in the adjustment during the regression analysis. All statistical analyses were performed using IBM SPSS 20.0 for Windows (IBM).
Reporting
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used for reporting observational studies(Reference Von, Altman and Egger32).
Results
From 1 January 2008 to 31 December 2018, 450 newborns weighing <1500 g were treated in our neonatal unit. Of these, fifty-nine died and in two cases data were not available on the nutrition received in the first week of life. Thus, a total of 389 newborns were finally considered for the study analysis (Fig. 1). The duration of enrolment of a child in the study cohort was 36 d (average; minimum 10 d, maximum 94 d). Table 1 shows the days of stay in the neonatal intensive care unit in each group and the maternal and neonatal characteristics of patients with and without BPD. As expected, except for the variables related to gestational age, there were no differences between the obstetric and maternal characteristics of newborns with and without BPD. Among the neonatal characteristics, and also as expected, the VLBW newborns who developed BPD had a lower weight, were more frequently males and were more likely to have a 5-min Apgar test score of < 7.
The incorporation of early PN in the standard protocol for the neonatal unit produced an increase in energy intake in the first week of life. Table 1 shows the weight z-score data in the first week of life. Although the energy intake was significantly lower in the BPD + group, the greater intake of liquids did not result in substantial weight differences in the first week of life. However, this increase in fluid intake was associated with a higher prevalence of PDA in the BPD + group.
Fig. 2 shows the annual prevalence of BPD in this period of time and the average energy intake received in the first week of life. We observed a statistically significant regression of the BPD prevalence and the energy intake in the first week of life in each year studied (R 2 0·48; P = 0·017). Apart from the energy intake provided, since BPD depends on more factors, newborns who developed BPD had a higher CRIB index at birth as can be seen in Table 1. As was also to be expected, the patients with BPD were more likely to present PDA, intraventricular haemorrhage, necrotising enterocolitis or sepsis co-morbidities, and as a result required a length of stay in the neonatal intensive care unit that was more than double that required by the newborns without BPD (Table 2). Table 3 shows the intake of fluids, energy content and macronutrients during the first week of life, for the patients who developed BPD and for those who did not. The weekly fluid intake per kg of body weight was significantly higher in patients with BPD (in calculating these values, the total intake obtained from EN and PN in the first 7 d of life was included). Also, infants with BPD had a lower intake of enteral feeds and a higher parenteral intake, which implies greater haemodynamic and respiratory instability that we know is associated with BPD.
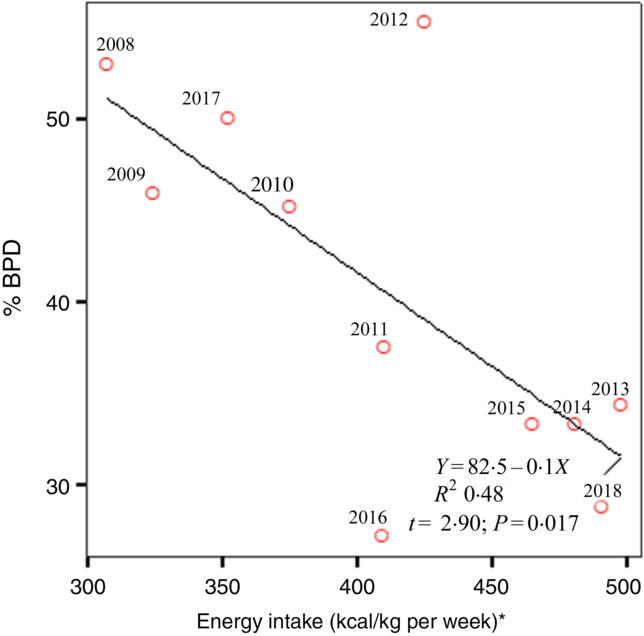
Fig. 2. Linear regression demonstrates the best line that predicts bronchopulmonary dysplasia (BPD) risk from average energy intake in the first week of life for the 11 years of the study. * To convert kcal to kJ, multiply the energy intake value by 4·184.
Table 2. Variables of respiratory support and associated co-morbidities in very low birth weight (VLBW) infants with and without bronchopulmonary dysplasia (BPD)
(Numbers and percentages; medians and interquartile ranges (IQR))
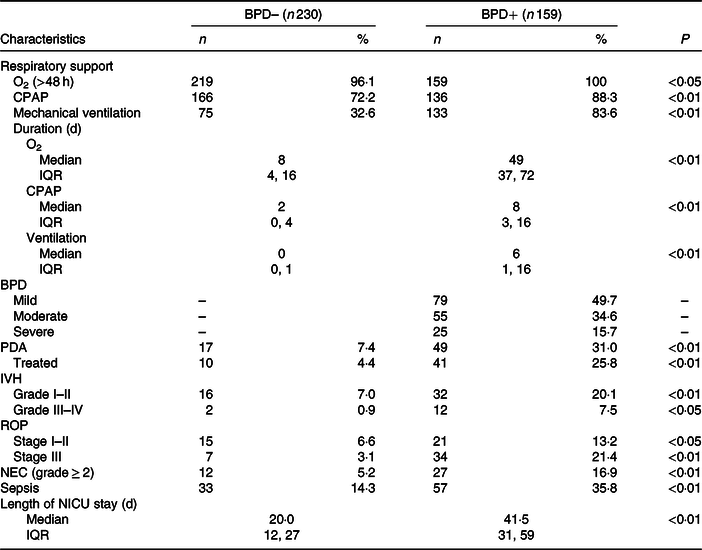
BPD–, without BPD; BPD+, with BPD; CPAP, continuous positive airway pressure; PDA, patent ductus arteriosus; IVH, intraventricular haemorrhage; ROP, retinopathy of prematurity; NEC, necrotising enterocolitis; NICU, neonatal intensive care unit.
Table 3. Liquids and nutritional support (enteral and parenteral) in the first week of life in very low birth weight (VLBW) newborns with and without bronchopulmonary dysplasia (BPD)
(Medians and interquartile ranges (IQR))
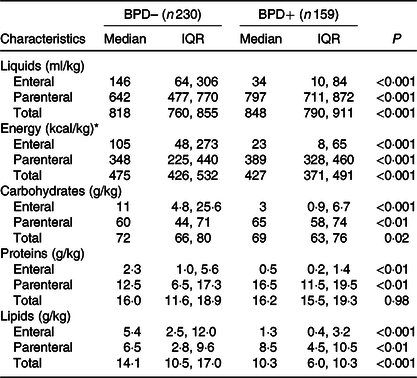
BPD–, without BPD; BPD+, with BPD.
* To convert kcal to kJ, multiply the energy intake value by 4·184.
Energy intake was significantly lower in patients who developed BPD. The 25th percentile of energy intake in the group without BPD practically coincided with the median of the group with BPD. Therefore, for the subsequent calculation of risk, this value (1778·2 kJ/kg per first week) was taken as the limit value. It is important to note that the greater energy intake obtained by the group without BPD was mainly due to a higher intake of fat. No differences in protein intake were observed between newborns with or without BPD, and the intake of carbohydrates was slightly smaller in the group with BPD.
Table 4 describes the neonates’ risk of BPD after receiving an energy intake in the first week of life less than that represented by the 25th percentile of the group without BPD. The OR values and the corresponding 95 % CI are shown both unadjusted and after adjusting for the differentiating variables identified at the outset (P < 0·05; see Table 1). The OR for BPD was 2·63 (95 % CI 1·30, 5·34) in the neonates who received an energy intake <1778·2 kJ/kg in the first week of life.
Table 4. Risk of bronchopulmonary dysplasia (BPD) and co-morbidities in very low birth weight (VLBW) newborns with energy intake lower than 25th percentile during the first week of life
(Odds ratios and 95 % confidence intervals)
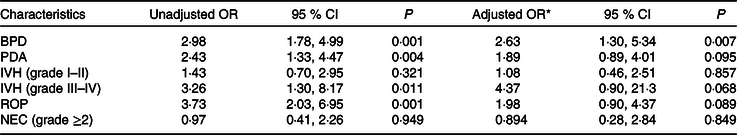
PDA, patent ductus arteriosus; IVH, intraventricular haemorrhage; ROP, retinopathy of prematurity; NEC, necrotising enterocolitis; IUGR, intra-uterine growth restriction.
* Adjusted for gestational age <27 weeks, male sex, chorioamnionitis, Apgar score, IUGR and human milk feeding.
Discussion
Our results show that an insufficient energy intake in the first week of life is associated with BPD development. This sufficient energy contribution can only be achieved in the premature newborn of very low weight by early initiating the contribution of lipids in PN solutions and when possible early EN.
Many factors may lead to undernutrition in patients with BPD, starting at birth with the cessation of transplacental nutrition of the fetus. The start of EN may then be delayed by the presence of haemodynamic and/or respiratory instability, generally related to the lower gestational age of the newborn. The early initiation of PN is often recommended in order to avoid energy catabolism in a newborn whose energy reserves are already diminished(Reference Bonsante, Iacobelli and Latorre14,Reference Ziegler16) . Inevitably, during the first days of life, there is an energy deficit, which is usually not corrected until the end of the first week of life. For this reason, and because the consequences of the energy deficit are cumulative, we quantified the overall energy provided in this period. Previous studies have reported that newborns with BPD have a lower growth rate than those with no BPD and that this difference extends beyond the period of hospital stay(Reference Klevebro, Westin and Stoltz33,Reference Wemhoner, Ortner and Tschirch34) . In our study, too, the newborns with BPD presented a decrease in the weight gain curve at 1 week of life, with significantly lower z-scores. In addition, energy malnutrition can compromise the repair of lung injuries associated with ventilotherapy, hampering normal lung development and reducing the efficiency of the muscles responsible for breathing(Reference Frank and Sosenko35). The sickest preterm infants develop BPD more frequently, as deduced from a higher CRIB score (Table 1). In these newborns, EN is often not possible, receiving energy intakes mainly as PN, as can be seen in Table 3. One might think that the lowest energy intake observed in newborns with BPD is because they are clinically more sick. However, the defect of enteral energy intake is replaced in most cases with parenteral energy intake. In addition, as can be seen in Fig. 2, it should not be forgotten that in our study, over the course of 11 years, several stages are passed, and in some of them with specific exceptions, energy intake in the first days of life was lower when the prevalence of BPD was higher. In this period of 11 years considered, there have been no substantial changes in the ventilatory management of these patients in our neonatal unit.
Studies have shown that early PN can be associated with less BPD, necrotising enterocolitis, retinopathy of prematurity and sepsis(Reference Hsiao, Tsai and Chen36,Reference Garcia-Serrano, Uberos and Anaya-Alaminos37) . This process stimulates endogenous insulin secretion, decreases the incidence of hyperkalaemia and promotes the secretion of insulin-like growth factors(Reference Larnkjaer, Molgaard and Michaelsen38). Some authors have associated an early increase in protein intake with a decrease in rates of BPD, while inadequate nutritional intake can lead critically ill newborns to present more co-morbidities and delayed postnatal growth(Reference Ehrenkranz, Das and Wrage9). In our study, no significant differences in the administration of protein were observed between newborns with or without BPD, although the energy intake received varied. In view of the results obtained for the average protein intake per kg of body weight in the first week of life, we believe the daily increase in this quantity might be insufficient.
In a preliminary cohort study(Reference Uberos, Lardon-Fernandez and Machado-Casas10), we could see that infants with BPD receive fewer enteral energy intakes in the first 14 d of life. That first study had a smaller size and did not allow clarifying conclusions. These preliminary findings were similar to those observed by other authors(Reference Wemhoner, Ortner and Tschirch39,Reference Malikiwi, Lee and Davies-Tuck40) , who report that premature newborns with BPD receive less enteral feeding, even when they receive nutrition that is well compensated for parenteral nutrient supply. For these authors, a minimum critical amount of enteral feeding is required to prevent the development of BPD. The current update of our cohort differs in many aspects from the previous report, especially because in our study we observe the importance of the energy intake as a whole, both EN and PN.
Fluid restriction is indicated in the first days of life, in order to reduce the possibility of the patient developing pulmonary oedema, PDA and BPD. However, this restriction, if the necessary precautions are not taken, can provoke undernutrition if the energy intake decreases correspondingly. In our opinion, in this process the early provision of lipids in the PN solution is fundamental to ensure an adequate energy intake.
Questions remain concerning the introduction of lipids in PN at an early stage. Some studies have concluded that no benefits are to be gained from the early administration of lipids in PN solutions(Reference Vlaardingerbroek and van Goudoever41,Reference Simmer and Rao42) . At one time, it was considered that in the VLBW newborn BPD increased in frequency and severity in parallel to the intake of lipids(Reference Hammerman and Aramburo43). It was also reported that an excessive supply of parenteral lipids exclusively from soyabean oil could be related to an increase in the levels of thromboxane B2 and other proinflammatory factors related to lung inflammation(Reference Sosenko, Rodriguez-Pierce and Bancalari44). A later study reported that an infant born at gestational age 27 weeks weighing 1000 g would present a severe deficit of DHA, which is thought to contribute to preterm morbidities such as BPD(Reference Lapillonne, Eleni dit and Kermorvant-Duchemin45).
Some randomised clinical trials have not observed reduction in BPD with parenteral solutions with a higher energy content(Reference Morgan, McGowan and Herwitker46) or reduction in lung morbidity with early PN or higher energy content(Reference Wilson, Cairns and Halliday47), although it might be objected that the sample size in both clinical trials may not be adequate. Klevebro et al., through a cohort study, observe that early provision of energy and protein may reduce postnatal weight loss and risk of BPD in extremely preterm infants(Reference Klevebro, Westin and Stoltz33).
We are aware of the limitations of our study as it is a retrospective cohort, although we believe that our data can help support future meta-analysis studies on this hypothesis. Strong evidence indicates that energy and protein intake in the first weeks of life is important in the neurological development of the newborn(Reference Vlaardingerbroek, Veldhorst and Spronk48,Reference Ohnishi, Ichiba and Tanaka49) . For this reason, the experimental designs under the clinical trial model, when the object of the study is the energy restriction in the premature newborn, may have ethical problems, so despite the biases, in our opinion, the cohort study model may be adequate.
In our study, diverse nutritional policies were recorded, including PN with soyabean oil exclusively (n-3:n-6 ratio of 1:7) or with added SMOFlipid (n-3:n-6 ratio of 2:5). Limiting the intake of lipids in PN to 3 g/kg per d is undoubtedly necessary in order to avoid the proinflammatory effects associated with excessive lipid intake. On the other hand, other authors have observed significant weight gains after the early introduction of lipids, from the first day of life, with these benefits persisting until 36 weeks’ corrected gestational age(Reference Fischer, Maucort-Boulch and Essomo Megnier-Mbo15). Lipid accretion within the fetus begins at approximately week 25 of pregnancy, which is why VLBW newborns have fewer deposits of long-chain PUFA. The n-3 and n-6 fatty acids are fundamental for the development of the brain and retina, and many studies have associated with decreased levels of long-chain PUFA in the VLBW newborn with delayed psychomotor development(Reference Vlaardingerbroek and van Goudoever41,Reference Hsiao, Tsai and Chen50) . Long-chain PUFA are known to modulate inflammation, and so early supplementation can reduce the incidence of BPD(Reference Manley, Makrides and Collins51), although other authors(Reference Collins, Makrides and McPhee52) have not observed a lower risk of BPD after enteral DHA supplementation.
Conclusions
The early nutrition and the increase of energy intake in the first week of life are associated in our sample with a lower risk of BPD developing. Without neglecting the protein intake necessary to prevent catabolism and to promote cellular anabolism, the early inclusion of lipids in the PN solution can be essential in order to ensure a sufficient energy intake is received, and thus to reduce the risk of BPD.
Acknowledgements
The authors thank the neonatologists and nursing staff involved for their invaluable collaboration.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. U. designed the analysis and interpretation of data and wrote the article and critically reviewed it for important intellectual content. He approves the version to be published. He agrees to be responsible for all aspects of the work to ensure that questions related to the accuracy or completeness of any part of the work are properly investigated and resolved. S. J.-M. and M. M.-O. designed substantial contribution to conception and design and wrote the article and critically reviewed it for important intellectual content. She approves the version to be published. She agrees to be responsible for all aspects of the work to ensure that questions related to the accuracy or completeness of any part of the work are properly investigated and resolved. J. L. G.-S. is responsible for the retinopathy of premature newborn programme, designed substantial contribution to acquisition of data and wrote the article and critically reviewed it for important intellectual content. He approves the version to be published. He agrees to be responsible for all aspects of the work to ensure that questions related to the accuracy or completeness of any part of the work are properly investigated and resolved.
The authors affirm that the work is original and is not being evaluated in any other journal. None of the authors has any conflicts of interest to declare.









