INTRODUCTION
During the last two decades the radiocarbon (14C) dating of hydroxyapatite archaeological cremated bones has become standard practice (Lanting et al. Reference Lanting, Aerts-Bijma and van der Plicht2001; van Strydonck et al. Reference van Strydonck, Boudin, Hoefkens and de Mulder2005; Olsen et al. Reference Olsen, Heinemeier, Bennike, Krause, Hornstrup and Thrane2008; Zazzo and Saliege Reference Zazzo and Saliege2011; De Mulder et al. Reference De Mulder, van Strydonck, Annaert and Boudin2012; Quarta et al. Reference Quarta, Tiberi, Rossi, Aprile, Braione, D’Elia, Ingravallo and Calcagnile2014; Agerskov Rose et al. Reference Agerskov Rose, Meadows, Palstra, Hamann, Boudin and Huels2019; Annaert et al. Reference Annaert, Boudin, Deforce, Ervynck, Haneca, Lentacker and Snoeck2020). The normal practice when considering bones for 14C dating is to avoid the inorganic bone fraction (hydroxyapatite) because of the risk diagenetic carbon exchange with e.g., dissolved carbonate ions (Lee-Thorp et al. Reference Lee-Thorp, Sealy and van der Merwe1989; Krueger Reference Krueger1991; Munro et al. Reference Munro, Longstaffe and White2007). However, Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001) demonstrated the that upon heating the crystallinity of the hydroxyapatite increases and as consequence hereof the hydroxyapatite becomes inert to diagenetic carbon exchange reactions. Thus for successful 14C dating of cremated bones, it is of vital importance to ensure that the cremated bones are burned at temperatures high enough for the re-crystallization process to have taken place (Shipman et al. Reference Shipman, Foster and Schoeninger1984; Stiner et al. Reference Stiner, Kuhn, Weiner and Bar-Yosef1995; Olsen et al. Reference Olsen, Heinemeier, Bennike, Krause, Hornstrup and Thrane2008). The crystallinity index (CI) representing a number between 3 (unburned bones) to 6–7 (bones burned at temperatures above ca. 600°C) can be measured using FTIR (Shipman et al. Reference Shipman, Foster and Schoeninger1984; Weiner and Bar-Yosef Reference Weiner and Bar-Yosef1990; Stiner et al. Reference Stiner, Kuhn, Weiner and Bar-Yosef1995). A more direct measure of the CI can be obtained using XRD (Person et al. Reference Person, Bocherens, Saliege, Zeitoun and Gérard1995).
Carbon stable isotope (δ13C) on hydroxyapatite from unburned bones lies typically around 15‰ representing the carbohydrate fraction of the diet (Lee-Thorp and van der Merwe Reference Lee-Thorp and van der Merwe1991). Intriguingly, the hydroxyapatite δ13C of cremated bones are much more 13C depleted with values around 25‰. This has led to interesting experiments from which it is concluded that during the heating process a large fraction of the hydroxyapatite carbon is exchanged with CO2 from the combustion fuel (Zazzo et al. Reference Zazzo, Saliege, Person and Boucher2009; Hüls et al. Reference Hüls, Nadeau, Grootes, Erlenkeuser and Andersen2010; van Strydonck et al. Reference van Strydonck, Boudin and de Mulder2010), i.e., measured 14C originates from wood in an archaeological context. This represents a possible complication when 14C dating cremated bones as the resulting 14C results may be influenced by the so-called old-wood effect (Olsen et al. Reference Olsen, Heinemeier, Hornstrup, Bennike and Thrane2013).
Various sample pretreatment methods exist for sample pretreatment of cremated bones (Lanting et al. Reference Lanting, Aerts-Bijma and van der Plicht2001; De Mulder et al. Reference De Mulder, Van Strydonck, Boudin, Leclercq, Paridaens and Warmenbol2007; Van Strydonck et al. Reference Van Strydonck, Boudin and De Mulder2009; Hüls et al. Reference Hüls, Nadeau, Grootes, Erlenkeuser and Andersen2010). The original method suggested by Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001) involves Sodium hypochlorite (NaOCl) to oxidize eventual organic material, then an Acetic acid (CH3COOH) step to remove readily soluble calcite, absorbed carbonates as well as the less crystalline fractions of hydroxyapatite. Subsequently the sample is homogenized and CO2 is produced by dissolving the cremated bone sample in phosphoric acid (H3PO4). As a last step the CO2 is furthermore heated with “Sulfix” particles (consisting of Ag2O and Co3O4, Waco Chemicals Ltd, LOT WAG 7038) to remove sulfur compounds. Some laboratories skip the organic matter oxidation step and use both hydrochloric acid (HCl) in combination with acetic acid and further re-combust the CO2 using silver wool and cupric oxide (CuO) as an alternative to Sulfix (De Mulder et al. Reference De Mulder, Van Strydonck, Boudin, Leclercq, Paridaens and Warmenbol2007; Van Strydonck et al. Reference Van Strydonck, Boudin and De Mulder2009; Hüls et al. Reference Hüls, Nadeau, Grootes, Erlenkeuser and Andersen2010). A recent study evaluated the pretreatment methods for cremated bones and concluded that no differences could be observed between the different pretreatment methods (Agerskov Rose et al. Reference Agerskov Rose, Meadows, Palstra, Hamann, Boudin and Huels2019). However, the latter study pointed out that the use of Sulfix could be problematic and might lead to inaccurate 14C results as also suggested by Zazzo et al. (Reference Zazzo, Lebon, Chiotti, Comby, Delqué-Količ, Nespoulet and Reiche2013). At Aarhus AMS Centre (AARAMS) we use the Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001) protocol for sample pretreatment and the reporting of the possible problems with the usage of Sulfix was therefore disturbing news. As part of a larger project, JOINTIME, the laboratory have 14C dated 31 cremated bones. We therefore decided to conduct a test comparing the CO2 purification methods by selecting 10 samples on which (1) the Sulfix and (2) the silver wool and CuO purification methods were used. This paper will report on the results of this test.
METHODS
Out of the 31 cremated bone samples which were 14C dated in the “JOINTIME: Connecting Bronze Age Europe: High-precision Radiocarbon Dating c.1700-1500 BCE” project 10 were selected for testing the CO2 purification method. The JOINTIME project focused on Bronze Age finds from the Eastern Carpathian Basin with the purpose of creating a high-precision chronology for the 1700–1500 BCE period. In the project strict sampling criteria were implemented, and in the case of cremated bones an effort was made to only pick white-chalky ones with a distinct glassy sound. Additional samples were at least one bronze object in their funerary inventory were preferred. Furthermore, the 10 samples used for the present test were selected from various geographic regions of the Eastern Carpathian Basin, in order to make the results representative for the entire region.
Two grams of bone sample is soaked in a 1.5% sodium hypochlorite solution to dissolve remaining organic material (48 hr, 20ºC). The sample is then washed and submerged in 1 M acetic acid to remove post-depositional carbonates as well as less crystalline, soluble fractions of bio-apatite (24 hr, 20ºC). Subsequently, the sample is washed and dried (12 hr, 80ºC) with a bio-apatite yield of approximately 96% calculated as the weight of the pretreated sample divided with by the weight prior to any chemistry. The pretreated sample is crushed and 1.5 g is treated with 85% phosphoric acid (8 hr, 80ºC) to liberate CO2. The CO2 aliquot was then split into two samples; where one being combusted with Sulfix for 2 hr at 470ºC and the other were combusted with CuO and Ag for 3 hr at 900ºC. The Sulfix material is preheated to 500ºC for 2 hr prior to its use. The two differently combusted CO2 aliquots were then reduced to graphite using hydrogen and Fe as a catalyst and pressed to targets for AMS analysis on the 1MV HVEE Tandetron accelerator (Olsen et al. Reference Olsen, Tikhomirov, Grosen, Heinemeier and Klein2017).
FTIR spectra were obtained using an Agilent Technologies Cary 630 ATR-FTIR instrument. Scans were performed in the range from 1500–450 cm1 with a resolution of 0.5 cm1. Each spectrum is an average of 64 scans. The spectra are baseline corrected and the C/P ratio is found using peak heights at 1415 cm1 and 1035 cm1, respectively. The CI is calculated after Olsen et al. (Reference Olsen, Heinemeier, Bennike, Krause, Hornstrup and Thrane2008).
RESULT AND DISCUSSION
From the 10 selected samples two pairs, AAR-31653 and AAR-31654 and also AAR-31656 and AAR-31657, are double burials from two different burial grounds: Voiteni (groapa de împrumut lut, Romania) and Vojlovica (Rafineria nafte-nekropola II, Serbia), respectively (Bukvić Reference Bukvić2000; Daróczi Reference Daróczi2015; Szentmiklosi Reference Szentmiklosi2021). In both instances, the remains of the two cremated individuals were placed in separate urns and the two urns were deposited in a single pit, clearly recognisable by a homogenous, undisturbed fill during the excavation (Daróczi et al. Reference Daróczi, Bălărie, Olsen and Birclin2023). Therefore, it can be expected that the 14C ages of these samples are identical. Detailed information on all 10 sampled burials has been published, in which the archaeological context and relative chronological value of their inventories has been analysed (Daróczi Reference Daróczi2021; Daróczi et al. Reference Daróczi, Rotea, Comşa, Olsen, Bârcă, Mustaţă, Lăzărescu, Rusu-Bolindeţ and Matei2022, Reference Daróczi, Bălărie, Olsen and Birclin2023).
All samples were visually inspected for burn cracks and showed white interiors suggesting high temperature burning (Figure 1). The sample pretreatment yield ranged between 83.2 to 95.6% (Table 1). The calculated CI range between 5.6 and 7.4 with a mean and standard deviation of 6.1 ± 0.5 confirming that all bone sample were cremated at temperatures above 600ºC (Table 1; Figure 2). Furthermore, the C/P ratios ranging between 0.02 and 0.06 (mean C/P ratio: 0.04 ± 0.01) suggesting a significant loss of carbon compared to unburned bones with a C/P ratio around 0.23 (Garvie-Lok et al. Reference Garvie-Lok, Varney and Katzenberg2004; Olsen et al. Reference Olsen, Heinemeier, Bennike, Krause, Hornstrup and Thrane2008).

Figure 1 All samples were visually inspected for surface and interior color and burn cracks as exemplified by the selected images (AAR-31620, AAR-31626, and AAR-31627) of the samples used in this study.
Table 1 Preparation yield, FTIR estimated C/P ratios and crystalinity index (CI) together with 14C results with different CO2 purification methods of either combustion with CuO and Ag or Sulfix. The ΔAge column denotes the difference in 14C years between the purification methods as well as the calculated z-score. For ΔAge the mean difference and standard deviation is calculated to –16 ± 32 14C years, whereas the weighted mean difference is –13 ± 12 14C years. The mean and standard deviation of the z-scores is calculated to –0.4 ± 0.8. Further, a reduced χ2 value of 0.9 is calculated with a χ2 limiting value of 1.9 (95% confidence).
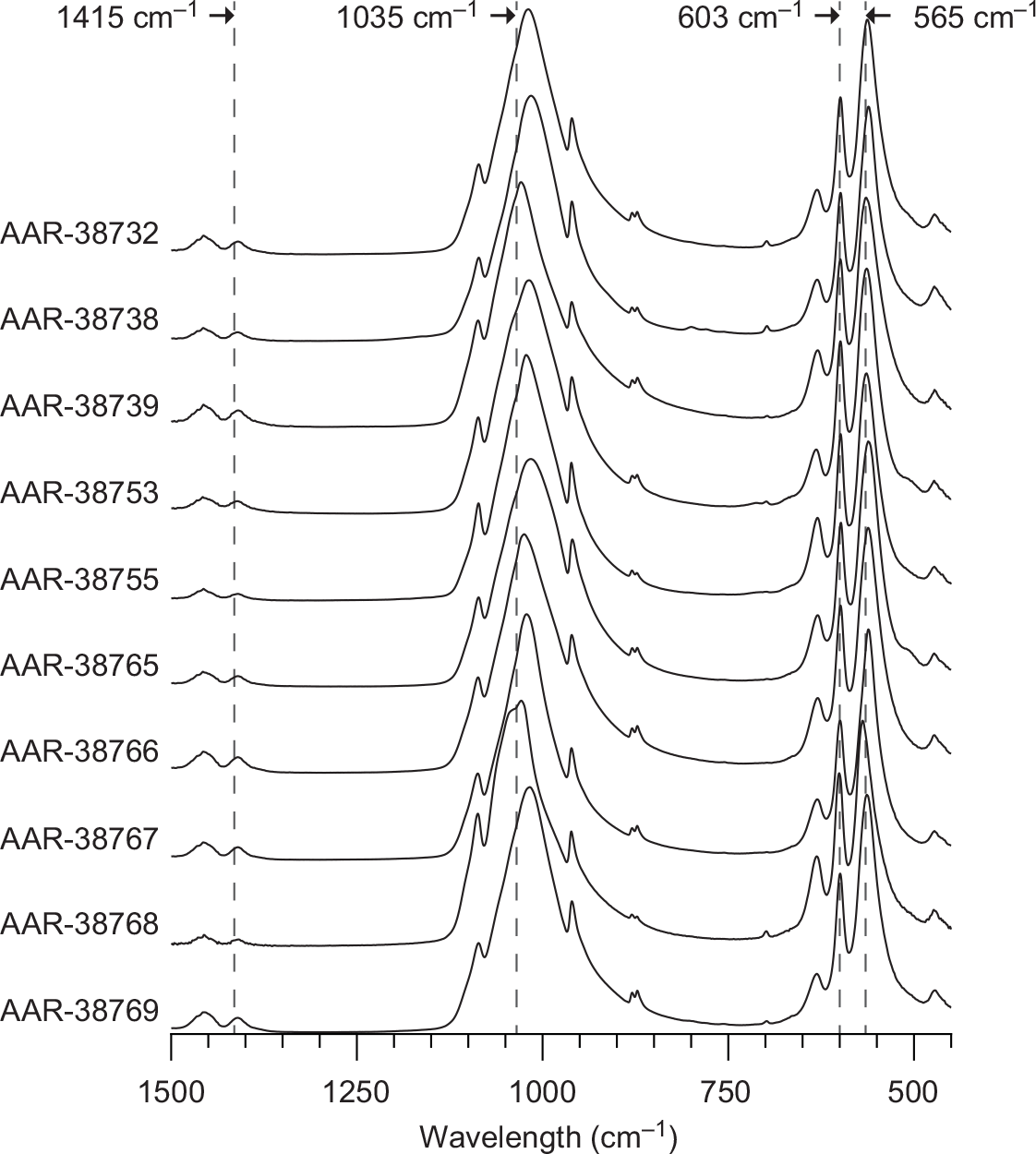
Figure 2 FTIR spectra of all samples used in this study. Marked are wavelength at 1415 and 1035 cm−1 representing the vibration bands of CO3 and PO4 respectively used to calculate the C/P ratio. The crystallinity index (CI) is a function of the extent of splitting of the two absorption bands at 603 and 565 cm−1.
The CO2 generated from dissolution in 85% phosphoric acid was split into two aliquots one for CO2 purification with CuO and Ag and another for CO2 purification with Sulfix. Hence, if the CO2 purification methods adds no further carbon to the sample, both CO2 fractions are expected to be similar within measurement uncertainties. The overall difference between the CuO and Ag and Sulfix CO2 purification methods are 16 ± 32 14C years (Table 1; Figure 3). The weighted average difference is calculated to 13 ± 12 14C years. The differences pass a reduced a χ2 test (reduced χ2: 0.9 ≤1.9) suggesting no outliers even though sample AAR-31656 shows a large z-score of 1.8 (Table 1). The reduced χ2 value of 0.9 further suggest the CO2 purification method differences to be normally distributed. Hence, the purification test indicates a small but systematic difference between the two purification methods where the Sulfix method appears to be slightly older than the CuO and Ag purification method.

Figure 3 Panel A shows the difference in 14C years between the CuO + Ag and Sulfix CO2 purification methods. The mean difference between the two methods is calculated to 16 ± 32 14C years. Panel B shows the calculated z-scores of the difference between the CuO + Ag and Sulfix CO2 purification methods. The z-scores mean is 0.4 ±0.9 and a χ2 test indicates that the difference is normally distributed (reduced χ2: 0.9 ≤1.9).
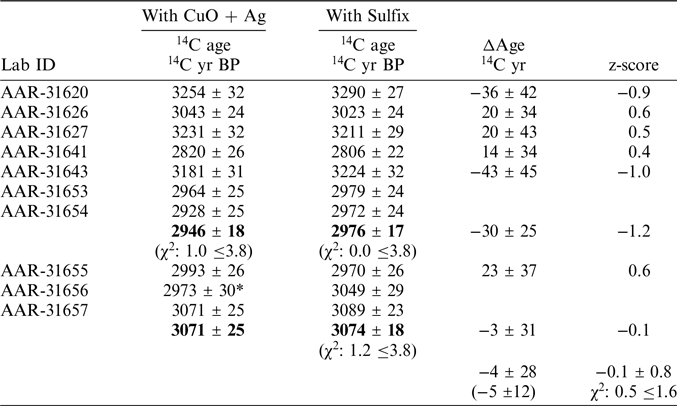
For the double burial (AAR-31653 with AAR-31654) the each of the purification methods can be combined to 2946 ± 18 14C years BP (χ2: 1.0 ≤3.8, Cu and Ag) and 2976 ± 17 14C years BP (χ2: 0.0 ≤3.8, Sulfix) respectively yielding a CO2 purification method difference of 30 ±25 14C years (Table 2). For the double burial AAR-31656 and AAR-31657 the CuO and Ag fractions cannot be statistically combined as the χ2 test fails (χ2: 6.3 ≤3.8). However, the Sulfix fractions can be combined to yield a combined 14C age of 3074 ± 18 14C years BP (χ2: 1.2 ≤3.8). Considering all 4 14C ages of the double burial AAR-31656 and AAR-31657 then the CuO and Ag fraction of AAR-31656 appears much younger and may thus be considered an outlier. Removing the CuO and Ag of sample AAR-31656 then the double burial AAR-31656 and AAR-31657 yield a CO2 purification method difference of 3 ± 31 14C years (Table 2). Using the updated CO2 purification methods differences the overall difference is calculated to 4 ± 28 14C years with a weighted average difference of 5 ± 12 14C years (Table 2). The differences pass a reduced a χ2 test suggesting no outliers (reduced χ2: 0.5 ≤1.6). The updated purification test, where the 14C ages of the double burials are combined, indicates a very small if not absent systematic difference between the two purification methods.
Table 2 CO2 purification test where 14C ages from double burials are combined.
CONCLUSION
Recently a study by Rose et al. (Reference Agerskov Rose, Meadows, Palstra, Hamann, Boudin and Huels2019) pointed out that the use of Sulfix for CO2 purification could be problematic and might lead to inaccurate 14C results. At Aarhus AMS Centre (AARAMS) we use the Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001) protocol for sample pretreatment and the reporting of the possible problems with the usage of Sulfix was therefore disturbing news. As part of a larger project, JOINTIME, the laboratory have 14C dated 31 cremated bones. Presented here a test comparing the CO2 purification methods by selecting 10 samples on which the Sulfix and silver wool and CuO purification methods are compared. Our CO2 purification test suggest a small and systematic difference between the CuO and Ag and Sulfix CO2 purification methods with the Sulfix purification method producing 14C ages being on average 13 ±12 14C years older than the CuO and Ag method. The difference is small and for the majority within measurement uncertainties, i.e., the systematic differences are within ±1σ. Thus, we consider the 14C ages obtained in the JOINTIME project to be valid. Taking into account that some of the chosen samples are double burials and therefore represents the same event. The 14C ages of the double burials where combined and the CO2 purification method difference were re-calculated yielding a purification method difference of 5 ± 12 14C years. Rose et al. (Reference Agerskov Rose, Meadows, Palstra, Hamann, Boudin and Huels2019) reported 14C age differences as large as 200 14C years, which was overcome by introducing a Sulfix precleaning step to remove the Sulfix carbon contamination. However, for future use we recommend to use a CO2 purification using CuO and Ag in order to avoid the possible complications associated with Sulfix. Because the supply of the Sulfix material is discontinued by Waco Chemicals Ltd the test presented here shows that other laboratories using Sulfix may use CuO and Ag as an alternative way of removing sulfur compounds evolving during the H3PO4 dissolution.
ACKNOWLEDGMENT
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Framework Programme under grant agreement no. 797494 (https://jointime.weebly.com/).










