INTRODUCTION
Infectious intestinal disease is common in childhood with each child probably experiencing four or more episodes of gastroenteritis before the age of 5 years. Rotaviruses, adenoviruses, astroviruses, noroviruses and sapoviruses are the major viral aetiological agents associated with childhood gastroenteritis.
Rotaviruses are members of the Reoviridae family and are classified into groups (A–E) on the basis of antigenic differences of the viral middle layer [Reference Estes and Knipe1]. Group A rotaviruses are the most common cause of human gastroenteritis, but groups B and C rotaviruses also infect humans. Group A rotaviruses are further classified into subgroups (SG) on the basis of immunological reactivities of the middle layer protein VP6, and into G- and P-types according to the diversity of the outer layer proteins VP7 (glycoprotein) and VP4 (protease-sensitive protein), respectively [Reference Estes and Knipe1]. To date, four different SGs (I, II, I+II and non-I/non-II), 15 G-types (G1–G15) and 20 P-types (P[1]–P[20]) have been identified among group A rotaviruses [Reference Estes and Knipe1].
Noroviruses (formerly known as Norwalk-like viruses or small round structured viruses) and sapoviruses (formerly known as Sapporo-like viruses) are members of the Caliciviridae family. Noroviruses are classified into three genogroups, and at least 16 genotypes [Reference Green2]. Similarly sapoviruses have been classified into three genogroups and several genotypes [Reference Schuffenecker3, Reference Dai4] although there is no firm agreement on the classification of these viruses as yet.
Astroviruses are members of the Astroviridae family and are classified into eight serotypes [Reference van Regenmortel5]. Human adenoviruses are members of the Adenoviridae family which are classified into six different subgroups or species (A–F) and within these into 51 distinct serotypes according to immunological, biochemical and biological differences [Reference van Regenmortel5]. Among these, adenoviruses of subgroup F, serotypes 40 and 41, have been associated with gastroenteritis and are termed enteric or fastidious adenoviruses [Reference Echevarria and Zuckerman6].
Electron microscopy (EM) has traditionally been used for the detection of enteric viruses in the faeces of infected individuals, however, this method is relatively insensitive, as detection requires ~106 virus particles/g faeces. This is a problem particularly for the detection of caliciviruses. Serological methods have been developed for the detection of some of these viruses. Enzyme immunosorbant assays (EIA) and passive particle agglutination tests (PPAT), some of which are available commercially, provide sensitivity comparable to EM for the detection of rotaviruses, noroviruses, astroviruses and adenoviruses. More recently, molecular methods, reverse transcription–polymerase chain reaction (RT–PCR) or PCR have been developed for the detection of enteric viruses [Reference Ando7–Reference Simpson10]. These methods provide improved sensitivity for the detection of all enteric viruses, but have had their greatest impact on the detection of human caliciviruses.
Although the association of these viruses with gastroenteritis is well established, the burden of disease associated with the different viruses is poorly described. Current data on the burden of disease are in the main derived from the routine voluntary reporting by clinical microbiology laboratories, and only a few structured surveillance studies have been undertaken in order to better define the burden of disease associated with the common viral pathogens [Reference Tompkins11–Reference Roman16]. Moreover, the differing sensitivities of detection methods used to diagnose enteric viral infections can produce a false picture of the incidence of infection with the different viruses. Many studies have used sensitive molecular methods for the detection of caliciviruses but EM or EIA to detect rotaviruses, adenoviruses and astroviruses. The use of techniques of lower sensitivity is highlighted by the ‘diagnostic gap’ which can result in more than 60% of gastrointestinal infection from which the aetiology is not determined. The use of molecular detection methods such as PCR can reduce the diagnostic gap to between 30% and 40% [Reference Simpson10].
The current study was undertaken in order to define the burden of viral gastroenteritis in children aged <6 years in the community, using sensitive molecular methods for the detection of rotaviruses, noroviruses, adenoviruses, astroviruses and sapoviruses. Data on disease severity and the cost associated with gastrointestinal disease obtained through detailed questionnaires completed by the parents/carers was collected and analysed in order to assess the severity of illness associated with the different viral agents of gastroenteritis, and to estimate the societal costs associated with an episode of infantile gastroenteritis.
MATERIALS AND METHODS
Patients and samples
Faecal samples collected in the winter months (December–April) during three consecutive winter seasons (2000–2003) from 685 patients aged <6 years with symptoms of gastroenteritis were tested for the presence of enteric viral pathogens. An episode of gastroenteritis was defined as acute onset of diarrhoea and vomiting or more than one episode of diarrhoea or vomiting within a 24-h period.
A total of 223 samples were from children recruited to a general practice-based structured surveillance study, of infantile gastroenteritis in the East Anglia region. The inclusion criteria were children aged <6 years who presented to a general practitioner (GP) with symptoms of gastroenteritis, diarrhoea and vomiting or diarrhoea. In addition, 203 and 259 samples were analysed from community and hospital-based cohorts, respectively. The samples from the community cohort were samples referred to Addenbrooke's Hospital, Cambridge by general practices in East Anglia not recruited to the structured surveillance, for the investigation of gastroenteritis. The hospital samples were from children with gastroenteritis admitted as in-patients or attending the A&E department at Addenbrooke's Hospital. For the hospital cohort, there was insufficient data to allow distinction between nosocomial cases and admissions due to gastroenteritis.
Data collection
Questionnaires were sent to the parents/carers of the children recruited to the structured surveillance study in order to collect data that would allow an estimation of the severity of illness and of the costs associated with gastrointestinal disease in this age group.
Parents were asked to complete a series of questions relating to the child's illness, medical care received, personal history, and socio-economic details (see Appendix). All information collected in the questionnaires was processed by computer in accordance with the Data Protection Act, 1990. Ethical approval for the study was obtained from the Regional Multicentre Research Ethics Committee.
The only data available for the non-structured surveillance cohorts was clinical diagnosis, age and sex.
Calculation of severity scores
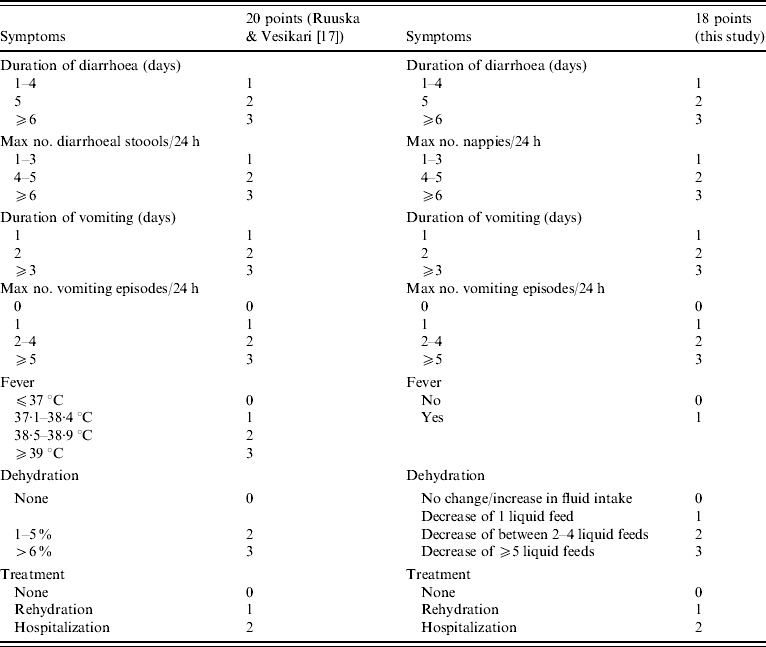
Parents/carers were asked to record parameters, which allowed us to assign a score to disease severity. Information on the quantity of liquid feeds prior to infection and during illness was collected, and this was used to determine the likelihood of dehydration. A significant reduction in the intake of fluids during illness in concert with diarrhoea and vomiting was regarded as an indicator of possible dehydration. Severity scores were calculated using a modification of the method of Ruuska & Vesikari [Reference Ruuska and Vesikari17]. Accurate temperature measurements were not available, therefore children with or without fever, reported by carers, were given scores of 1 and 0 respectively. This resulted in a maximum score of 18 points rather than the 20 originally described (Table 1). An episode of gastroenteritis is considered mild when the severity score is ⩽5, moderate when severity scores range between 6 and 10, and severe when the severity scores are >10.
Table 1. Criteria used for calculating disease severity scores; comparison with previously published criteria
Virological investigations
Faecal samples were prepared as 10% suspensions in balanced salt solution (M199, Sigma, Poole, Dorset, UK) and stored at 4°C. Nucleic acid was extracted using guanidinium isothiocyanate/silica and cDNA synthesized in the presence of random primers [Reference Boom18, Reference Iturriza-Gomara19] The cDNA was used in PCRs for the detection of enteric adenoviruses, astroviruses, noroviruses, group A rotaviruses, and sapoviruses using oligonucleotide primers and methods described previously [Reference Simpson10]. Also, a PCR for the detection of group C rotaviruses was optimized and used in this study. Oligonucleotide primers were designed to amplify a region of the gene encoding the VP6 [RV-CVP6F: 5′-ATW GAA GCY GTA TGT GAT G-3′ (nt 296-314) and RV-CVP6R: 5′-ATT RTT TGG TGC ATT YTC AG-3′ (nt 615-634)]. Rotavirus-positive samples were genotyped using method described previously [Reference Bermingham20].
Statistical analysis
χ2 test and Student's t test were performed to determine the significance of differences observed between the different groups of patients and/or incidence of infection with the different viruses (STATS™ v.1.1, Decision Analyst ’98, Arlington, TX, USA).
RESULTS
A viral aetiological agent was detected in 367/685 (53·6%) samples tested (Table 1). A viral pathogen was detected in a significantly greater number of patients from the structured surveillance group than from either the community or the hospital cohort (P<0·01) and also in the community cohort than in the hospital cohort (P<0·05).
Rotavirus was the most common viral pathogen found in all three groups, followed by norovirus and enteric adenoviruses (Table 2). No significant differences were observed in the relative incidences of the different viruses between the three cohorts.
Table 2. Virus distribution in cases of gastroenteritis (including multiple infections)
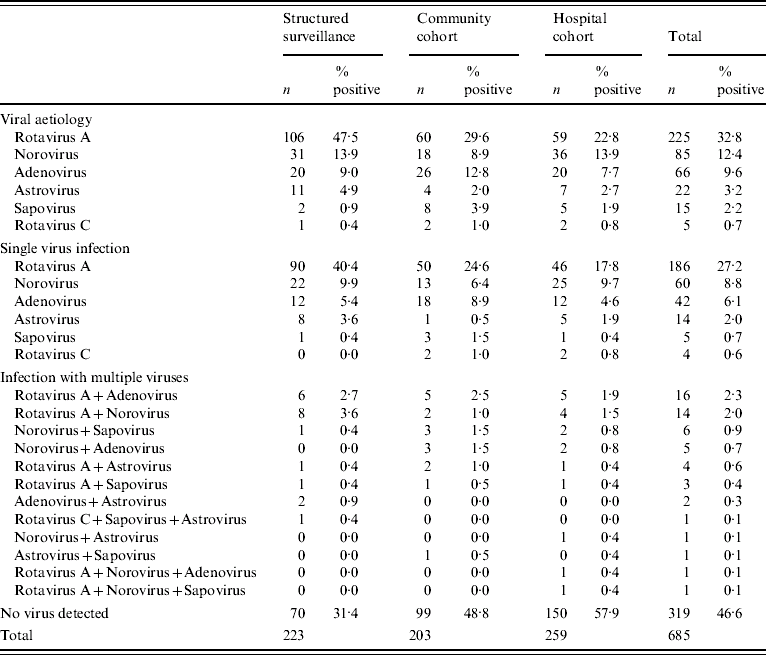
Multiple viral pathogens were found in 55/686 (8%) of the samples. Among these, two viral pathogens were found in 53 samples, and three viral pathogens in a further two samples (Table 2). Multiple infections involving rotavirus and any other virus were most common (n=40), followed by norovirus with any other virus (n=28) and adenovirus with other viruses (n=24) (Table 2).
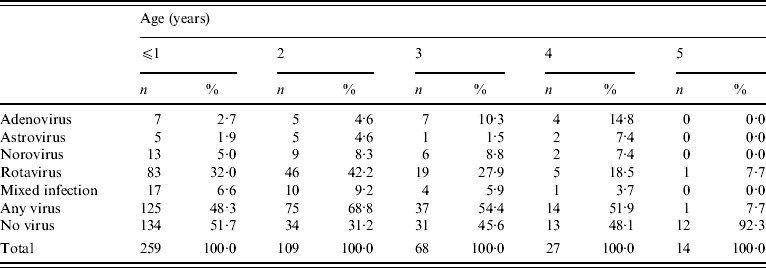
Although samples were collected from children aged ⩽5 years, they were predominantly from children aged ⩽2 years. The numbers of children presenting with gastroenteritis to their GPs declined as age increased.
Rotavirus was the most common viral pathogen in all age groups, followed by norovirus in ⩽1- and 2-year-olds, and enteric adenovirus in 3- and 4-year-olds (Table 3). No significant differences were seen in the incidence of mixed infections between age groups (Table 3). In children aged ⩽1 year the number of samples in which no viral pathogen was identified was significantly higher in the hospital cohort compared to the same age group in the structured surveillance and community cohorts (hospital cohort, 75%; structured surveillance cohort, 37·4%; community cohort, 45·6%; P<0·01) and also compared to any other age groups (P<0·01) (excluding the 5-year-olds as the numbers of children in this group were insufficient for meaningful comparison). In the structured surveillance cohort, the mean age of children for which a pathogen was identified was the same as that of children for whom no aetiological agent was identified, 1·9 years, and the median age was 2 and 1 years, respectively.
Table 3. Virus distribution by age
Rotavirus genotyping
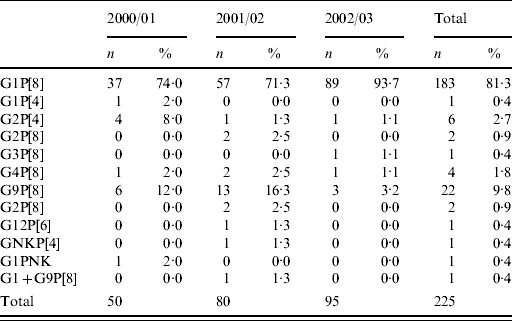
G1P[8] was the most commonly detected rotavirus strain in the structured surveillance group and the community and hospital cohorts and there were no significant differences among rotavirus genotypes detected in each patient group.
Temporal distribution indicated that although G1P[8] was the most frequently found rotavirus each year, significantly higher numbers of G1P[8] strains were found during the 2002/2003 season than in the previous two seasons. This was accompanied by a decrease in the number of infections with G9P[8] strains detected in 2002/2003 with respect to the two previous seasons (Table 4). A G12P[6] rotavirus strain was detected for the first time in the United Kingdom. This strain failed to amplify in the G-type-specific multiplex second-round PCR, and was characterized through sequencing of the first-round amplicon.
Table 4. Distribution of G- and P-type combinations of 225 rotavirus-positive samples collected from 2000 to 2003 (including structured surveillance, community and hospital cohort samples)
Severity scores were available for 49 patients from whom a rotavirus was detected and genotyped. The majority (n=37) were G1P[8] and severity scores ranged from 6 (mild) to 16 (severe).
Structured surveillance: analysis of data obtained through completed questionnaires
A total of 142 (64%) completed questionnaires were returned of which 136 provided sufficient information on disease severity to be evaluable.
Days between onset of symptoms and sample collection
There was no statistically significant difference between the means of days after onset when samples were collected from children with or without an identified viral aetiology. The mean number of days between onset of symptoms and sample collection was 5·4 days (median 5) for samples from which a viral pathogen was identified, and 6·1 days (median 5) for samples from which no viral pathogen was detected.
Severity of illness
In total there were 136 respondents providing information on the duration and number of episodes of diarrhoea and vomiting, the presence or absence of fever and the use of oral rehydration therapy which allowed the calculation of severity scores (Table 5).
Table 5. Severity score determined for 136 patients with gastroenteritis

Infections with enteric adenoviruses, and astroviruses were more often associated with mild to moderate disease, and severe disease was more frequently associated with noroviruses and rotaviruses; however, infections of moderate or severe disease were associated with all viruses with the exception of astrovirus (Table 5). The median severity scores for children aged from <1 to 2 years were 10, compared with scores of 9, 8, and 7·5 for children aged 3, 4 and 5 years, respectively. The median severity score for patients from whom no virus was detected was 7 (range 3–15). There was no statistically significant increase or decrease in severity associated with astrovirus, adenovirus or norovirus infections (P>0·05). Rotavirus infections were statistically more likely to be more severe than any other virus infection or in symptomatic patients from whom no aetiological agent was found (P=0·0014) (Table 5). The analysis of the symptoms reported showed associations between rotavirus and norovirus infections and the duration and number of episodes of vomiting and contributed to the higher severity scores associated with rotavirus infection. Children from whom a viral pathogen was identified were more likely to require rehydration therapy than those with gastroenteritis of unknown aetiology (P<0·01) (Table 5).
DISCUSSION
Previous studies of the burden of infectious intestinal disease associated with gastroenteritis viruses indicated that, in children aged <5 years, rotaviruses or noroviruses are the major causes of infectious gastroenteritis in this age group [Reference Tompkins11–Reference Roman16]. Incidences of rotavirus and norovirus infection among different studies ranged from 9·7% to 32·0% and from 2% to 20·2%, respectively. Adenoviruses, astroviruses and sapoviruses were each responsible for <10% of gastrointestinal infections in all studies (Table 6).
Table 6. Comparison of structured surveillance studies of infantile gastroenteritis
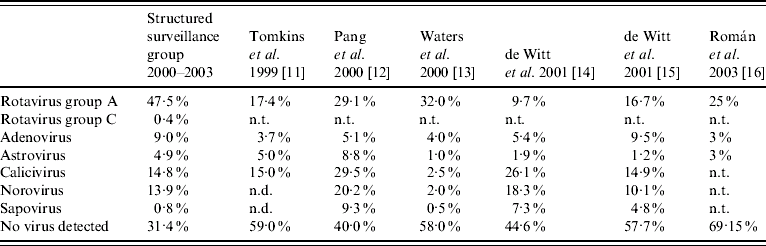
n.d., Not differentiated; n.t., not tested.
Detection methods of low or differing sensitivities were used in all of these studies and may have influenced the incidence rates determined for each viral pathogen. In the current study, sensitive molecular methods were used to detect rotaviruses (groups A–C), adenoviruses types 40 and 41, noroviruses, sapoviruses and astroviruses. It has previously been shown that the sensitivity of detection can be increased by 22–700% depending on the viral pathogen sought [Reference Simpson10].
Analysis of the results of all samples (structured surveillance cohort and community and hospital cohorts) showed that a virus or viruses were identified in 53·5% of samples. As in other reported studies, rotaviruses and noroviruses were associated with the majority of infections. Interestingly, analysis of the structured surveillance cohort alone showed that a virus was identified in 68·6% of samples. This difference may be associated with a firmer case definition used in the structured surveillance study including, acute onset of diarrhoea and vomiting or more than one episode of diarrhoea or vomiting in a 24-h period whereas controls were selected on a clinical diagnosis of ‘gastroenteritis’, ‘diarrhoea and vomiting’ or ‘diarrhoea’. Therefore, the community and hospital cohorts may have included children with chronic symptoms or non-infectious diarrhoeas or the lack of detection may reflect a time-lag between the onset of illness and sample collection.
No differences were observed in the distribution of rotavirus genotypes between the structured surveillance and the community or hospital cohorts and in all groups G1P[8] were the predominant strains. A rotavirus G12P[6] was detected for the first time in the United Kingdom from one of the patients in the community cohort. G12 strains in combination with either P[6] or P[9] have recently been described in sporadic cases of infantile diarrhoea in several parts of the world [Reference Das21–Reference Shinozaki24]. G9P[8] strains which emerged in the United Kingdom in 1995 were found throughout the study, but their incidence decreased during the last year of the study. Previously we observed that these viruses first emerged in the United Kingdom predominantly in urban areas and that the incidence of infection with G9 strains increased in the first two years in the geographical areas where they were detected to become the second most common strain, followed by a significant decrease in their incidence during the third year [Reference Iturriza-Gomara25, Reference Asmah26]. At the time G9 rotaviruses were first found in the United Kingdom in 1995/1996 to 1998/1999 they were not detected in the eastern region of the United Kingdom, which is mostly a rural area. The results may suggest that G9P[8] strains emerged in East Anglia later, and the incidence of infection with these viruses peaked in 2001/2002, decreasing subsequently.
The use of numerical scores for disease severity allows comparisons among studies and of different aetiological agents within studies and allows the whole range of symptoms associated with viral gastroenteritis to be taken into account. Median severity scores decreased with age and this probably reflects the impact of immunity, acquired previously, which modified the illness making it less severe rather than preventing infection and disease. Infections with rotaviruses and noroviruses were associated with higher severity scores than other viral agents and were similar to those described by Pang et al. [Reference Pang12]. In a limited study by Roman et al., in which only tests for rotavirus, adenovirus and astrovirus were included, rotavirus infections again had the highest severity score [Reference Roman16].
Severity scores in this study (between 6·5 and 11) and the study of Pang et al. (between 5 and 10) [Reference Pang12], indicated mild or moderately severe disease associated with cases of gastroenteritis in the community. The duration of diarrhoea ranged from a median of 5 days for norovirus and astrovirus to 6 days for rotavirus, whereas, in the study of Pang et al., the duration of astrovirus-associated diarrhoea had a median of 1 day, rotavirus 4 days and adenovirus 5 days [Reference Pang12].
There were no significant differences between this study and the study of Pang et al. [Reference Pang12] when the number of diarrhoea episodes, the duration of vomiting, or the number of vomiting episodes were compared. In both studies, vomiting was significantly associated with rotavirus infection and both rotavirus and norovirus infections produced the highest numbers of vomiting episodes in a 24-h period.
Interestingly, children infected with adenovirus were less likely to require rehydration than children infected with any other virus, this may be directly associated with the finding that adenovirus infections were more prevalent in older children. Rehydration therapy is likely to be required more often and be more complicated in children of low weight, which includes the very young and the malnourished.
In comparison to previous studies in which virus detection included EM or EIA and PCR for selected virus targets, the current study used molecular methods only. There was no significant increase in the detection of caliciviruses among studies as molecular methods were used in all. The detection of rotaviruses was enhanced through the use of molecular methods with a significantly higher incidence of rotavirus infection identified in the current study. Also, the ‘diagnostic gap’ was reduced to 31·4% through the use of molecular methods in conjunction with structured surveillance. Interestingly, the median time from illness to the collection of a faecal sample was 5 days and might suggest that duration of illness as well as severity of symptoms are significant causes to seek medical attention. This late sample collection is likely to impact on the ability to detect viral pathogens, and if common, it would suggest that the more sensitive molecular detection methods should be used for diagnosis.
The ability to close further the ‘diagnostic gap’ may involve the improved detection of other enteric pathogens including bacteria and parasites. All the samples in the community and hospital cohorts, and in the first year of the current study, and all structured surveillance samples were submitted for routine enteric bacterial investigation, but no significant non-viral pathogens were found to cause disease in this age group. However, in subsequent years, two children recruited to the structured surveillance reported having been diagnosed with Campylobacter infection. Similar studies have found bacteria such as Salmonella spp., Campylobacter spp., Shigella spp., Yersinia spp. and verotoxigenic Escherichia coli to be the aetiological agent associated with gastroenteritis in a total of 11·9% of cases in this age group [Reference de Wit15]. Similarly, parasites such as Cryptosporidium, Giardia and Entamoeba have been associated with 7·7% of infectious enteric disease in young children [Reference de Wit15].
Viral pathogens such as toroviruses, kobuviruses and parvoviruses, not sought routinely, may be associated with a proportion of the undiagnosed cases. Waters et al., detected toroviruses by EM in 3% of cases of community-acquired diarrhoea in children [Reference Waters13]. In the current study, all samples from the structured surveillance cohort were negative for torovirus by RT–PCR (data not shown).
With the exception of adenoviruses, the common gastroenteric viral pathogens have RNA genomes, which during replication have high mutation rates. This results in virus families of wide antigenic and genomic diversity, and it may be difficult to construct a single assay capable of detecting all members of the virus family. Antibody-escape mutants and mutations that result in detection failures in molecular assays have been described for rotaviruses [Reference Iturriza Gómara27–Reference Iturriza-Gomara, Kang and Gray29].
ACKNOWLEDGEMENTS
This project was funded by the NHS Executive Eastern Region, Research and Development Directorate (HSR/1199/6).
DECLARATION OF INTEREST
None.
APPENDIX. Questionnaire sent to parents/carers












