Introduction
Humans eat meals with complex combinations of nutrients and non-nutrients that are likely to interact because of their biochemistry, metabolism and utilisation for a range of biological processes. Many of these dietary components are able to impact brain function and may converge on similar biological pathways. Much of the scientific interest and efforts on the effects of nutrition on brain function have focused on increasing understanding of the role of individual food bioactives, such as long-chain omega-3 polyunsaturated fatty acids (PUFA)(Reference Dacks, Shineman and Fillit1), B vitamins(Reference Clarke2), antioxidants such as vitamins C and E(Reference Mangialasche, Kivipelto and Mecocci3), carotenoids(Reference Li, Shen and Ji4) and polyphenols(Reference Joseph, Cole and Head5–Reference Lehtisalo, Levalahti and Lindstrom10).
However, diet is complex owing to thousands of different nutrients which act in additive, antagonistic and/or synergistic ways. This complex interaction amongst nutritional components is one reason why nutritional research is and should be clearly distinguished from pharmaceutical research that typically investigates the isolated actions of one molecule during a specific and identified issue as opposed to normal age-related decline. Typically, foods and nutrients (e.g. fatty acids, polyphenols, etc.) are consumed in varying proportions and combinations, rather than in isolation. Therefore, it might be speculated that the effects of nutrients could be underestimated by disregarding potential interactions with other nutritional components. For example, observational studies of ageing cohorts have identified numerous dietary patterns linked to the slowing of cognitive decline (e.g. the Mediterranean-style diet), which has led to the suggestion that specific nutrients may be key modulators of this beneficial effect(Reference Scarmeas, Anastasiou and Yannakoulia11).
Nonetheless, interventional studies, in which individuals are supplemented with one of these bioactives, often fail to show a clear positive effect of the component on cognition(Reference Scarmeas, Anastasiou and Yannakoulia11). There may be several reasons for this, for example, differences in statistical power and timescales. However, one important factor, considered here, is that synergistic and antagonistic interactions can occur between nutrients and food, making the ‘whole’ very different from the sum of its parts. The effects observed in the observational studies may be due to a sum of the effect of many nutrients contained within a specific dietary pattern, which cannot be reduced to one or two key elements. This raises questions regarding the approach to adopt when designing multi-nutrient intervention trials. Indeed, whilst there is a general agreement that a combination of nutrients may potentially result in more potent and more relevant benefits for brain functions, there is currently no consensus as to suitable research approaches to examine the effect of nutrients or non-nutrient combinations. Here we consider the challenge of studying the impact of combining nutrients and food bioactives on brain health, with the aim of producing recommendations for future research and providing recommendations based on good scientific practice. We hope these recommendations will provide food for thought for other researchers.
Considerations for research designs aiming at investigating the effects of food component combinations on brain functions
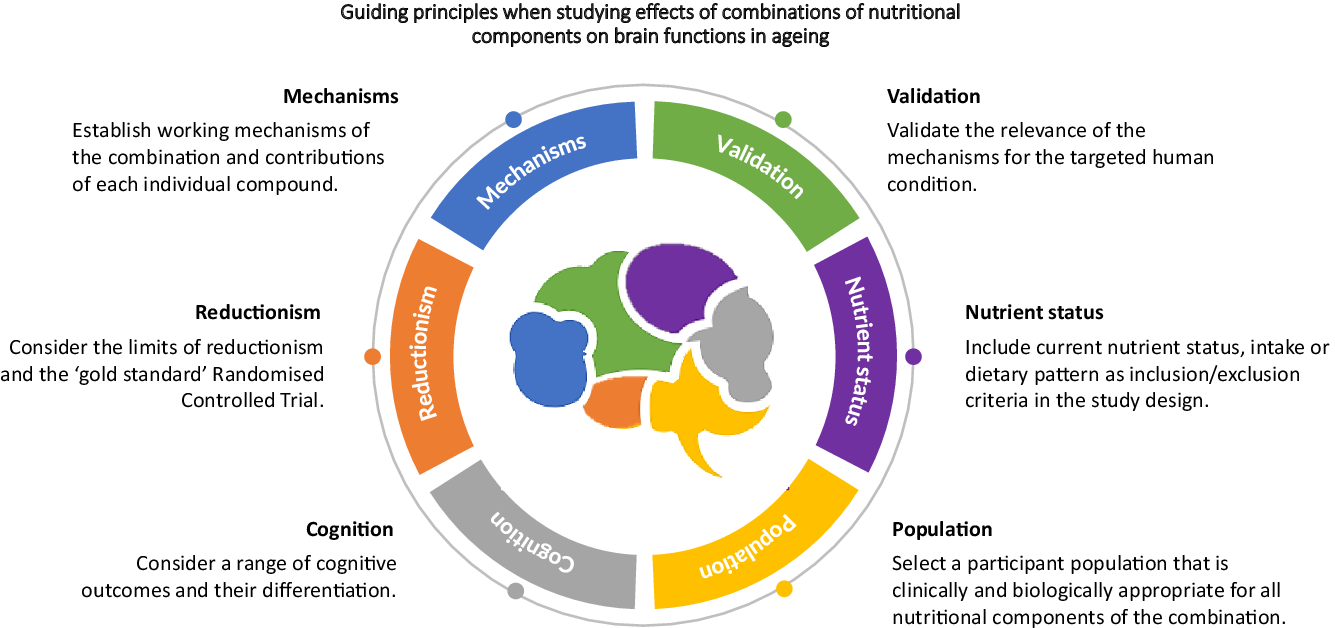
Establish working mechanisms of the combination and contributions of each individual compound
In the past decade, the effect of nutrition and dietary components on brain structure and function has been investigated intensively. This research has shown that dietary patterns, and their impact on possible mechanisms such as neuroinflammation and cerebro/cardiovascular impairment (e.g. chronic cerebral hypoperfusion), may influence the development of particular types of dementia such as Alzheimer’s disease (AD)(Reference Gardener, Scarmeas and Gu12–Reference Kennedy, Wightman and Reay21). The combination of dietary components that simultaneously, and possibly synergistically, target an underlying risk factor and/or physiological process could have an even larger effect on inhibiting the development of neurodegenerative disorders than single nutrients targeting that process(Reference Joris, Mensink and Adam17,Reference Gorelick, Scuteri and Black22) . Therefore, one approach is to identify working mechanisms of food component interactions, and how the contribution of each individual compound may affect those mechanisms.
Dietary nutritional components may influence numerous biological pathways which could be beneficial at the cellular and systems level. Although a single ingredient may be able to influence such a pathway, it is likely that food component combination may provide a better result than any compound in isolation. Previous studies have identified effects of dietary nutrients on several specific cellular pathways that have systemic effects. For example, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) found in high concentration in oily fish and fish oil have shown in both in vitro brain cell culture experiments and in vivo animal studies to mitigate inflammatory pathways, through activation of peroxisome proliferator-activated receptor γ (PPARγ)(Reference Mita, Beaulieu and Field23) and the NF-κb transcription factor(Reference Paterniti, Impellizzeri and Di Paola24), both of which are involved in many biological pathways including inflammation. Notably, other nutrients such as glutamine, selenium, spices or flavonoids are also able to influence PPARγ and NF-κB(Reference Marion-Letellier, Dechelotte and Iacucci25,Reference Youn, Lim and Choi26) , indicating the potential for additive or synergistic effects.
Indeed, the clinical evidence for the beneficial effects of DHA supplementation on cognitive decline is mixed(Reference Wu, Ding and Wu27), suggesting that long-chain omega-3 PUFA may need to be given in combination with additional nutrients to synergistically produce an effect at the cellular and systems level. In agreement with this scenario, one example is the VITACOG study in which the effect of B vitamins on slowing down cognitive decline is enhanced when omega-3 fatty acid concentrations are in the upper range(Reference Oulhaj, Jerneren and Refsum28). Moreover, antioxidant nutrients can also protect the highly susceptible long-chain omega-3 PUFA from peroxidation when co-supplemented. Accordingly, it has been shown that response to omega-3 PUFA supplementation was enhanced in individuals when consumed with dark-green vegetables which are good dietary sources of folate, lutein, carotenoid, vitamin K and vitamin B6, suggesting a potential interaction between these nutrients and the absorption or bioavailability of omega-3 PUFA(Reference O’Sullivan, Armstrong and Schuster29–Reference Harris and Von Schacky33).
Another type of interaction could involve activating a detoxification pathway. For example, pairing red meat with fresh garlic containing allicin was able to block dietary carnitine derived trimethylamine production in the gut(Reference Barabási, Menichetti and Loscalzo34). This ultimately leads to lowering of the trimethylamine N-oxide (TMAO) concentration in plasma, therefore protecting against the development of cardiovascular diseases. Notably, other components found in the Mediterranean diet such as wine, olive oil and grapeseed oil are also able to lower TMAO(Reference Wang, Klipfell and Bennett35–Reference Wu, Panyod and Ho38). Indeed, one advantage of a nutritional approach to improve brain health is that, unlike specific pharmacological approaches, dietary interventions have the possibility to include numerous components simultaneously that may act in synergy(Reference Kardum and Glibetic39–Reference Zhang, Zheng and Liu41). Nonetheless, it is equally important to recognise that nutrients may also have antagonistic effects on one or multiple pathways of interest, or impact the absorption of each other, making the understanding of a combinatorial approach more challenging than expected. For example, dietary polyphenols such as flavonols, anthocyanins and isoflavones exert many positive actions, including anti-proliferative, anti-inflammatory, immuno-regulatory and neuroprotective effects(Reference Forbes-Hernandez, Gasparrini and Afrin42). However, when they are ingested with macronutrients, the bioavailability and bioactivity can be significantly changed. Polyphenols such as those found in coffee or tea are often consumed with milk. The binding of polyphenols to milk proteins negatively affects the bioefficacy and may decrease, for example, vascular protective effects and antioxidative properties of polyphenols(Reference Kardum and Glibetic39–Reference Zhang, Zheng and Liu41). Polyphenols themselves affect the uptake and bioavailability of vitamins and minerals, especially iron, zinc and calcium(Reference Cianciosi, Forbes-Hernandez and Regolo43,Reference Jakobek and Matić44) . For example, in Wistar rats it has been demonstrated that polyphenolic extracts of green tea, chokeberry or honeysuckle fruits decrease zinc and calcium absorption(Reference Frejnagel and Wroblewska45) .
In conclusion, rather than focusing on specific nutrients, it may be more advantageous to concentrate instead on identifying specific mechanisms. It may then be possible to find a group of ingredients that influence such pathways leading to desirable behavioural outcomes(Reference Kardum and Glibetic39–Reference Zhang, Zheng and Liu41,Reference Frejnagel and Wroblewska45–Reference Wurtman48) . Nonetheless, to design a multi-nutrient approach it is important to understand the contribution of each individual component on the hypothesised working mechanism to favour synergistic effects(Reference McNamara, Kalt and Shidler49). A caveat is that whole diets include dozens of foods providing thousands of molecules, which results in complex biological activity. Such complex systems may not simply dissect into constituent parts. Focusing on one or two of these molecules or potential pathways means that the vast majority are ignored when they may have active influence. The consequence is unpredictable ‘emergent’ properties. Therefore, whilst it may be possible to define isolated functions and linear contributions of some nutrients in some small part of the system, the approach is likely to be limited, and complementary approaches (discussed later) may be required.
Validate the relevance of the mechanisms for the targeted human condition
Often, neurological diseases are influenced by biological pathways, including those outside of the brain, which could be targeted with nutrition interventions to enable greater treatment efficacy. However, verifying the association between specific biological mechanisms and cognitive and brain health is crucial. For example, it was reported that type II diabetes is a risk factor for developing AD(Reference Sridhar, Lakshmi and Nagamani50,Reference Steen, Terry and Rivera51) . Both conditions are associated with impaired glucose metabolism and insulin resistance(Reference Zhao and Townsend52). Therefore, assuming that insulin resistance is a contributing factor to the pathogenesis of AD, therapeutic interventions aiming to improve insulin resistance in combination with brain-specific pathways may be beneficial. A second example comes from recent work indicating a possible relationship between blood pressure and AD pathology(Reference Arvanitakis, Capuano and Lamar53). Remarkably, a report from the SPRINT MIND trial indicated that intensive blood pressure reduction in older adults resulted in a significant reduction in the number of individuals developing mild cognitive impairment over the 8-year study(Reference Williamson and Pajewski54). This suggests that dietary intervention to reduce blood pressure throughout the lifespan, coupled with nutrients targeting the brain, may act in cooperation to reduce the risk of developing dementia. However, often biological pathways are poorly understood and causal links to the behavioural outcome are missing. When using biological mechanisms to inform the design of multi-nutrient trials, it is necessary to differentiate risk factors from mere correlates. While causal risk factors can be manipulated to change the probability that an outcome will occur, correlates cannot. One reason for non-significant results in nutrition trials based on prior significant epidemiological observations might be a failure to differentiate between risk factors for the development of disease and correlates that change because of the disease process. Therefore, validating the relevance of biological pathways for the human condition is a key aspect that needs to be addressed early on, to have real synergies or even additive outcomes.
Biology is complex; however, some modifiable risk factors for cognitive decline and dementia have been identified. For example, changes in plasma levels of the amino acid homocysteine is considered a risk factor for dementia, and related changes in brain structure(Reference Seshadri, Beiser and Selhub55). There are two pathways by which homocysteine is broken down: re-methylation that requires folate and vitamin B12, and the trans-sulphuration pathway, which requires vitamin B6. A supplement of these three co-enzymes was given for 2 years and reduced the levels of homocysteine(Reference Seshadri, Beiser and Selhub55). Structural MRI was used to measure any increase in the size of the ventricles, a reflection of the shrinking of the brain. The rate at which the brain was shrinking was less in those taking the vitamin supplement. These findings illustrate that, if multi-nutrient interventions target well-validated causal pathways, beneficial behavioural outcome can be observed.
An example of a successful multi-component approach is the specific formulation of nutrients, registered as Fortasyn Connect. Owing to the additive effects of the combination of DHA, uridine, choline, folate, vitamin B12, vitamin B6, vitamin E, vitamin C and selenium(Reference Beeri, Haroutunian and Schmeidler46,Reference Chen, Mecca and Naganawa47) , this supplement promotes synaptogenesis(Reference Wurtman48) to counteract the synaptic loss occurring in cognitive impairment and AD. These ingredients may act via several synergistic mechanisms including, but not limited to, providing precursors for the Kennedy cycle, lowering homocysteine levels, and increasing cerebral blood flow and perfusion(Reference Rasmussen56). Many of these nutrients are also common in the Mediterranean diet, for example, fish oil (DHA), tomatoes (uridine), choline in eggs, fish and nuts, and vitamins and antioxidants in onions, lentils and beans. The Mediterranean diet has been found to have numerous beneficial physiological effects, including reducing blood pressure(Reference Filippou, Thomopoulos and Kouremeti57) preventing type 2 diabetes and improving glycaemic control(Reference Esposito, Maiorino and Ceriello58), and lowering inflammation(Reference Bonaccio, Pounis and Cerletti59) and cholesterol levels(Reference Esposito, Maiorino and Bellastella60). Notably, these are all well-established prospective risk factors that may increase the odds of developing dementia in a dose-dependent way. It was reported that, compared with no risk factors, the relative risk for dementia was 1·20 for one risk factor, 1·65 for two and 2·21 for three or more risk factors(Reference Peters, Booth and Rockwood61). Therefore, the combination of these dietary components found in the Mediterranean diet and Fortasyn Connect may protect against cognitive decline, and ultimately AD, by targeting inflammation, stimulating neuronal cell membrane production and improving vascular and metabolic health, which cannot be achieved by single ingredients/dietary components by themselves(Reference Scarmeas, Stern and Tang62,Reference Bianchi, Herrera and Laura63) . It should, however, be noted that in one of the main Fortasyn Connect trials the effect on the primary endpoint (change in a neuropsychological test battery (NTB; composite z-score based on the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) ten-word list learning immediate recall, CERAD ten-word delayed recall, CERAD ten-word recognition, category fluency, and letter digit substitution test) was not significant, dividing scientific opinion regarding the effectiveness of this multi-nutrient formula in those with mild cognitive impairment(Reference Soininen, Solomon and Visser64).
It is also important to consider that other multi-nutrient studies have failed to demonstrate synergistic or additive effects on cognition. For example, as mentioned previously, the combination of fish oil and anthocyanin-rich blueberries in older adults did not interact to produce cognitive enhancement(Reference McNamara, Kalt and Shidler49). Instead, those who consumed blueberries alone had an improvement in discrimination memory(Reference McNamara, Kalt and Shidler49). The authors speculated that the combined treatment may have subverted the beneficial effect that was observed when blueberries were consumed only by excessively upregulating the transcription factor NF-E-2 related factor 2. This finding emphasises how, in complex systems, nutrients may not always interact in a predictable way, highlighting the need for a clear understanding of the proposed mechanistic synergies to produce positive outcomes.
Metabolomics
The targeting of specific pathways is usually based on previous knowledge about the relationship between the risk factor and outcome. However, a limitation of such an approach is that it fails to adequately capture the complexity of the metabolic response to nutrients present in the human diet and the consequences for cognitive health. This might explain some disappointing or counter-intuitive findings that have emerged when multi-nutrient trials have targeted isolated mechanisms. Consequently, recent attention has been directed towards data-driven approaches such as metabolomics. This method combines measuring all small molecules present in a biological system with multi-variate statistics enabling the discrimination of a specific ‘metabolic profile’ which characterises a physiological state or response to an intervention. The benefit is the potential identification of new markers of nutrient intake associated with health and disease. Such an approach might be particularly useful for understanding complex interactions of nutritional metabolites.
For example, biomarkers have been identified for macro-nutrients, food groups and dietary patterns such as a vegetarian, omnivorous or Mediterranean diet(Reference Guasch-Ferre, Bhupathiraju and Hu65). In addition, an attempt was made to find molecular markers in the blood of those with dementia: thirty-three metabolites that differed from the healthy elderly were identified(Reference Teruya, Chen and Kondoh66). For example, reduced levels of ergothioneine, a potent antioxidant, were found in those with mild cognitive impairment. Some oxidoreductants found in erythrocytes, including NADP+, glutathione, adenosine triphosphate, pantothenate, S-adenosyl-methionine and gluconate, were lower in those with dementia. These findings are, however, correlational, and it remains to be established that they have a role in the development of dementia and, if so, whether diet can play a role.
Nonetheless, the predictive utility of the approach has been demonstrated in a prospective cohort studied over 12 years. Those over 65 years who did not show signs of cognitive decline were compared with those who did(Reference Low, Lefevre-Arbogast and Gonzalez-Dominguez67). Baseline levels of twenty-two metabolites were found to distinguish these groups, including three coffee metabolites, a biomarker of citrus, a cocoa metabolite and metabolites from fish and wine. Adding these measures to a model that predicted cognitive decline, and that included variables such as ApoE-ϵ4, diabetes and BMI, increased its predictive ability.
Although the approach has great potential, the answers are unlikely to be easy. For example, about 18 690 metabolites have been established in human blood, never mind other bodily tissue. It should also be remembered that metabolomic research is in its infancy, and many identified biomarkers have not yet been related to clinical outcomes. Although metabolomics promises to add to our understanding of the influence of diet, until relevant data become available, findings should be cautiously interpreted.
Overall, there is potential to improve the efficacy of nutritional interventions by combining nutrients that target complementary pathways known to predict cognitive decline and dementia. However, whilst some studies have produced very promising results, others have been disappointing. This highlights the complexity with which dietary and systemic mechanisms interact. Metabolomics hold promise for capturing the complexity of the metabolic response to nutrients. An important factor in driving this area forward will be a clearer understanding and better validation of the main biological pathways and/or ‘metabolic profiles’ that play a causative role in cognitive ageing to facilitate the design of multi-nutrient interventions.
Include current nutrient status, intake or dietary pattern as inclusion/exclusion criteria in the study design
One key consideration should be to include current nutrient status, intake or dietary pattern as an inclusion/exclusion criterion in the study design. Indeed nutrient–nutrient interactions may involve an interaction between the experimental nutrient, and the diet habitually consumed by the individual/population. Therefore, in intervention studies, it is important to quantify the intake of relevant nutrients in the individuals’ diet. Not doing so risks masking any possible beneficial effects of the nutrient supplementation, and limits understanding of the ability to metabolise the nutrients in the specific population. For example, interventional studies in older adults involving dietary supplementation with omega-3 PUFA have produced less convincing and more mixed results(Reference Baleztena, Ruiz-Canela and Sayon-Orea68,Reference Vakhapova, Cohen and Richter69) ; for review, see Weiser et al. (Reference Weiser, Butt and Mohajeri70). For example, in the OmegAD cohort, supplementation with DHA and EPA for 6 months did not delay the rate of cognitive decline(Reference Freund-Levi, Eriksdotter-Jonhagen and Cederholm71). However, in a follow-up analysis the authors report an inverse correlation between plasma levels of omega-3 PUFA and the rate of cognitive decline(Reference Eriksdotter, Vedin and Falahati72). Furthermore, in the Multidomain Alzheimer Preventive Trial (MAPT), DHA and EPA supplementation over 3 years had no significant effects on cognition in elderly individuals(Reference Andrieu, Guyonnet and Coley8). Like the OmegaAD study, the authors also noted that individuals with lower levels of DHA and EPA in blood exhibited a larger decline in cognitive measures than those individuals with normal concentrations. Together these data suggest that systemic levels of DHA may be important for slowing cognitive decline. However, as noted above, supplementation with the single compound, or in combination with EPA, may not be enough, as the beneficial effects of omega-3 fatty acids may be due to interactions with existing factors within the diet. Selection of the population should be guided towards individuals with low intake of foods containing all the components of interest.
Regarding habitual diet, individual variation in microbiota and microbiome is also an important aspect to consider. Much attention is now being focused on the bidirectional communication between gut microbiota and the brain, particularly with regard to host brain activity modulation via a microbiome–gut–brain axis(Reference Bastiaanssen, Cowan and Claesson73). For example, an increase in the microbial load and the metabolites released can increase the permeability of the intestinal barrier, allowing the stimulation of innate immunity cells by lipopolysaccharide. The subsequent circulation of pro-inflammatory cytokines or short-chain fatty acids derived from bacterial metabolism(Reference Schönfeld and Wojtczak74) may affect the blood–brain barrier and lead to their entry into the brain(Reference Borre, O’Keeffe and Clarke75), where they stimulate microglial cells and cause neuroinflammation. The products of bacterial metabolism and pro-inflammatory mediators produced after immune stimulation induced by the microbiota can reach the brain through a leaky blood–brain barrier.
It is now recognised that foods and nutrients influence the microbiome in different ways. For instance, a Western diet with high fat and sugar intake reduces the microbial diversity and affects cognition by triggering chronic low-grade inflammation(Reference Komanduri, Gondalia and Scholey76–Reference Proctor, Thiennimitr and Chattipakorn79). In addition, essential fats, proteins, non-digestible carbohydrates, probiotics and polyphenols all induce shifts in the microbiome with consequences for host metabolic and immunologic markers(Reference Singh, Chang and Yan80) Therefore, attempts to investigate effects of a specific diet in a population already adhering to that diet may be of low yield and/or limited by small changes in their microbial diversity. Notably, data from the Personalised Responses to Dietary Composition Trial (PREDICT) indicated that the gut microbiome composition predicted several post-prandial glycaemic, lipaemic and inflammatory indices(Reference Berry, Valdes and Drew81). In addition, the nature of intestinal microbiota contributes to the inter-individual variations in the metabolism of various phytochemicals(Reference Setchell, Brown and Lydeking-Olsen82). Essentially, an individual’s response to a food, or a group of foods, is likely to depend on the pre-existing composition of their microbiome. Therefore, for dietary interventions based on nutrients, foods and dietary patterns, the participants’ background dietary intake along with the consideration of the baseline microbiota is critical.
Select a participant population that is clinically and biologically appropriate for all nutritional components of the combination
Disease stage
Those interested in nutrient–nutrient interactions in the context of ageing will also need to consider that the aspects of nutrition examined will depend on the stage of disease; some aspects of nutrition, such as the Mediterranean diet, will influence risk factors such as inflammation or oxidative stress, prior to the development of a disorder. If subsequently a disease develops, different aspects of nutrition may be influential. For example, whereas dietary interventions that maintain a steady supply of glucose to the brain might facilitate memory in healthy adults(Reference Young and Benton83), a decline in cerebral glucose metabolism in Alzheimer’s disease could diminish such benefits. Instead, diets that induce ketosis, and hence provide alternative source of energy for the brain, might be beneficial(Reference Cunnane, Trushina and Morland84). Therefore, it is conceivable that some nutritional components are more effective before clinical disease onset and others may have shown benefits in subjects who already manifest disease symptoms. As a result, selection of the nutritional components to be studied in combination will partially depend on the disease stage of our population.
An interesting example of dietary interaction is that the nature of the diet consumed during the early formative years may ameliorate the deleterious effect of poor later nutrition, for example by increasing cognitive reserve(Reference Benton85). Therefore, the aspects of nutrition of interest will depend not only on the stage of the disease, but also the time frame over which nutrients interact. Some of the nutritional components of the combination may have neurobiological activity within relatively shorter time frames, while other nutritional components may require considerably longer periods to be able to observe a meaningful cognitive effect. As discussed below, prospective epidemiology offers a potential means to study these challenging questions. For example, the Lothian Birth Cohort 1936 is a rare example of a cohort which has assessed cognition during childhood (age 11 years) and again in older age (age 70+ years). Using this dataset, Corley et al. (2020) found that the strength of the association between a Mediterranean dietary pattern and cognition was attenuated by 50% after controlling for intelligence at age 11 years(Reference Corley, Cox and Taylor86). Additionally, prospective cohort studies that have repeatedly over decades assessed diet, for example, the Avon Longitudinal Study of Parents and Children (ALSPAC)(Reference Emmett87), allow the evaluation of multiple nutritional components over time. In addition, longitudinal cohort studies may allow effects to be considered on hard clinical endpoints. In contrast, RCTs are often limited to intermediate outcomes or lack the power to identify disease states that over a short period will occur rarely.
Phenotype
Selecting a group of subjects with homogeneous phenotypes is another important aspect. It is reasonable to expect differential responses to different nutrients across the different phenotypes (which in turn may refer to different underlying biopathological entities). In the context of dementia, although a high percentage of subjects will have AD or vascular dementia, there are multiple other different possible aetiologies. A dietary intervention tailored for a specific disease aetiology is unlikely to have impact across a range of aetiologies; for example, in a clinical trial, vitamin E, vitamin C and α-lipoic acid influenced markers of CSF oxidative stress, but not amyloidosis or neurodegeneration(Reference Galasko, Peskind and Clark88). Thus, in multi-component nutritional interventions it is paramount to carefully select nutritional components referable to a phenotypically and biologically homogeneous population group. Imaging, genetic profiling, medical assessment and biochemical data may help create a sample of participants with a common aetiology. It should be noted that, whilst an important consideration, the selection of a very homogeneous sample will limit the generalisability of any positive research findings.
Age and sex
We know that effects of nutrition on brain function may depend on many parameters, including age, sex and predispositions to various conditions (i.e. metabolic syndrome). For example, a high intake of PUFA has been found to be associated with a slower annual cognitive decline in the oldest spectrum of a population (i.e. age 73–91 years), but not in individuals aged 65–72 years(Reference Vercambre, Grodstein and Kang89). Vitamin D status has been shown to have a closer relationship with cognitive decline in older women, compared with older men(Reference Breitling, Perna and Muller90). Low intake of saturated and trans lipids and higher intake of PUFA may have a more profound beneficial effect on cognitive decline in individuals with diabetes(Reference Devore, Stampfer and Breteler91). The above become more important when more than one nutritional component is examined. For example, it is conceivable that one component may be more effective in younger rather than older individuals, whilst another component preferentially benefits those who are older. One diet may be more efficacious in men and another in women. Therefore, it is important to tailor our selection of nutritional components to the characteristics of the targeted population.
Genotype
Similarly, genetic subgroups may be important to consider when studying nutrient combinations. It is probable that a growing question will be how foods interact with specific genes to increase or decrease the risk of cognitive dysfunction. Nutrigenetics, how genetics influences the response of the body to a nutrient, needs to be considered. Such responses may be different for different components of a nutrient combination used within a single study. For example, interactions with APOE status have been reported for many nutrients. Studies have revealed that the omega-3 fatty acid intake may be more pertinent for preventing cognitive decline in those individuals carrying at least one of the APOE ϵ4 alleles(Reference van de Rest, Wang and Barnes92,Reference Nooyens, van Gelder and Bueno-de-Mesquita93) . As there could be an APOE (or other genetic) interaction with one nutrient, but not another, of a multi-component nutritional formulation, the genetic structure of the target population may be central. Given that cognition is polygenetic, genome-wide association studies, from which polygenic scores can be calculated(Reference Aronica, Volek and Poff94), might prove profitable in the future.
To summarise, to enhance our understanding of nutrient–nutrient interactions, there may be a need to move away from the ‘one-size-fits-all’ approach. Although the individual differences discussed above are not exhaustive, it is clear that the treatment and prevention of disease via nutritional combinations may need to also consider the variability in lifestyle, genetics, microbiome, demographics and environment of groups of individuals or, ideally, each individual.
Consider a range of cognitive outcomes
Many symptoms of the ageing of the brain are cognitive. The assessment of cognition raises a range of general methodological issues that have been reviewed previously(Reference Devore, Stampfer and Breteler91), although when considering several nutrients at the same time there is additional complexity. Cognitive functioning clusters into six major domains: executive functioning, memory, attention, perception, psychomotor and language. Each domain can be further subdivided: for example, memory, amongst other distinctions, includes short-term, long-term and working memory, and involves processes such as encoding, consolidation and retrieval. You may be interested in a particular aspect of cognition, but there may be unpredictable outcomes such that other aspects of cognition or mood, that were not being experimentally examined, are influenced. Given the complexity of the diet and the complexity of the brain, the ‘law of unintended consequences’ comes into play.
Varying several aspects of nutrition may influence the functioning of several areas of the brain and hence different aspects of its functioning; thus, changes in a measure of one aspect of cognition may in practice reflect changes in another. Without measuring a range of aspects of cognition, you cannot know if any change reflects an improvement in the one aspect of cognition that is of interest, rather than another aspect of functioning that facilitates the first. An example is an association between stress, depression and memory. A systematic review of those with mild memory problems found that those with anxiety, but not depressive symptoms, were later more likely to become cognitively impaired(Reference Desai, Whitfield and Said95). When under stress, the body releases cortisol, and over time an area of the brain associated with memory, the hippocampus, shrinks(Reference Ouanes and Popp96). Therefore, when studying memory as a measure of cognitive decline, we need to also monitor stress. There is no point in addressing the neural mechanisms associated with memory if the problem is stress related. We must address the cause and not the consequences.
A need to consider a range of aspects of brain functioning is illustrated by a review of forty-six studies that found a higher intake of flavonoids improved depression(Reference Ali, Corbi and Maes97), although it has also been said that ‘flavonoids may exert particularly powerful actions on mammalian cognition and. may reverse age-related declines in memory’(Reference Spencer98). Unless you monitor both, how can you be sure that a dietary-induced memory improvement is any more than better mood? The message is that we need to monitor a range of aspects of cognition functioning and the more that aspects of diet are varied the greater the likelihood that a range of measures will be influenced. The performance of a psychological test demands an ability to maintain attention, remember, and develop strategies (executive functioning), motivation, mood and language skills. If you measure only one of these mechanisms, the way diet was influential will be unclear, a consideration that becomes more important when several aspects of diet are being examined.
The problem of examining a single primary outcome is illustrated by studies that were hoping to improve memory. A post-hoc analysis of the Multi-domain Alzheimer Preventive Trial (MAPT) found that supplementation with DHA, in older individuals with low blood omega-3 fatty acids, benefited executive functioning but no other cognitive domain(Reference Zwilling, Talukdar and Zamroziewicz99), that is, the expected change in memory was not observed. Similarly, in older individuals, the frequent consumption of seafood for five years was associated with a lesser decline in semantic memory and perceptual speed, but not episodic or working memory, visual spatial functioning or global cognition(Reference Breitling, Perna and Muller90). This was an unexpected finding as the initial memory problems associated with dementia reflect episodic rather than semantic memory(Reference Prentice and Huang100). If there are unpredictable consequences with one nutrient, then the potential is greater when many nutrients are combined, as they may interact in nonlinear ways. Thus, a consideration of psychological phenomena demands a test battery that assesses all cognitive domains that are potentially influential.
Only if we show that an improvement in functioning reflects memory as such, and not a change in mood, arousal, attention, executive functioning or anything else, will we have established that an intervention has influenced disease progression. Clearly, any positive response to an intervention is to be welcomed. However, unless you benefit memory as such, you are not slowing the progress of a disease; rather, you are optimising any residual capacity, but only while some capacity remains. The take-home message is that cognition, in general, should be measured rather than only memory or some composite measure that adds together things with little in common. Of course, when measuring multiple outcomes, appropriate corrections to prevent inflating the changes of type 1 error, should also be applied.
Consider the limits of reductionism and the ‘gold standard’ randomised controlled trial
There is a well-established and rigorous way of designing experiments: you vary an independent variable and predict the consequences for a dependent variable, stating a priori the primary outcome measure. Although this approach will hopefully be used in this area, in some cases it might not be the most efficient way of proceeding. The problem is complexity which results from the need for detailed study of the linear and non-linear interactions between various parameters. The present state of knowledge makes it difficult to develop precise predictions. Therefore, although randomised controlled trials (RCT) are considered the ‘gold standard’, when it comes to dietary interventions, we may need to acknowledge that well-conducted epidemiological studies have several advantages(Reference Lichtenstein, Petersen and Barger101). In fact, there is substantial evidence from epidemiology that many aspects of diet are associated with the risk of dementia. One explanation is that observational prospective studies benefit from the ability to assess the aggregate effects of multiple nutrients over an extended period – something especially pertinent when considering the effects of diet on the development of dementia(Reference Benton85). For example, by combining data from three longitudinal cohorts, Moore et al.(Reference Moore, Ames and Mander102) observed that those with low plasma concentrations of vitamin B12 and high concentrations of folate in the blood have a substantially higher risk of cognitive impairment compared with those with low blood folate and low plasma vitamin B12. Therefore, observational data may also be able to address questions concerning the influence of multiple nutrient deficiencies that may occur with age, questions unlikely to be assessed in RCT owing to practical and ethical considerations. Additionally, Zwilling et al. (2019) combined nutrient biomarker pattern analysis with functional brain network analysis and cognitive testing. It was reported that high plasma ω-3 and ω-6 PUFAs, lycopene, carotenoids and vitamins B (riboflavin, folate, B12) and D were associated with better cognitive functioning(Reference Zwilling, Talukdar and Zamroziewicz99). Furthermore, plasma levels of ω-3 and ω-6 PUFAs and lycopene moderated the association between brain network connectivity and cognition. This study illustrates how innovative methods from epidemiology, cognitive and network neuroscience can be combined to enhance understanding of how nutrient interactions might influence brain ageing.
Importantly, epidemiology tends not to look at single nutrients, or specific foods, but rather at dietary styles combining a range of nutrients. The advantage of this approach is that it might help capture the complexity of nutrient–nutrient interactions, although establishing the active molecules and the mechanism of action is difficult. Without such an understanding, precise dietary recommendations are not possible, and the development of functional foods is precluded when it is unlikely that any benefit will reflect a single nutrient or food. We need to know if the benefit of a style of eating reflects the small influence of many foods and, if so, which ones. If there are synergistic, additive or antagonist effects, which foods are involved? Additionally, a thorough understanding of nutritional epidemiology is required to ensure that research delivers reliable and robust results on which to base the development of RCTs. A complete discussion of epidemiological methods is beyond the scope of this article and can be found elsewhere(Reference Prentice and Huang100,Reference Almirall, McCaffrey and Ramchand103–Reference Fairchild and McDaniel106) . Dietary pattern analysis is considered in the section: decomposing the complexity of diet.
In performing a multi-nutrient intervention study there are many considerations to consider. Broadly speaking, interventions can incorporate the multi-nutrient dimension in one of two ways: through a food-based approach where foods enriched in the components are consumed as part of a daily diet or in a capsule form where the components are incorporated into the capsule. In the latter scenario, the control group receives an intervention identical in appearance and sensory characteristics and blinding is easy to implement. The biggest disadvantage is the loss of the food matrix and potential contributions(Reference Aguilera107,Reference Wahlqvist108) . With respect to whole diets and food-based approaches, it is more difficult to develop a control intervention identical to the test intervention minus the multi-nutrient bioactive components. Furthermore, blinding is more difficult but not impossible. There are examples of food-based interventions that have successfully used a food substitution approach to alter multiple components of the diet in a controlled fashion(Reference Weech, Vafeiadou and Hasaj109,Reference Tierney, McMonagle and Shaw110) . Notwithstanding the limitations of either approach, positive response to a nutritional intervention can be interpreted only in comparison with the group with which it is compared. Thus, the negative consequences of the control diet need to be as clearly defined as the active intervention. It is as useful to conclude that there is a negative response to a poor diet as well as a beneficial response to a good diet.
To conclude, such is the importance of RCTs that, when claiming a causal relationship, epidemiological data can be downplayed. However, for practical and ethical reasons there are many situations where it is not possible to run a RCT. It is not questioned that smoking is a risk factor for lung cancer, although nobody has randomly required half their subjects to smoke for a lifetime. Similarly, nobody is going to randomly allocate people to a diet that is suggested to increase the risk of dementia.
Decomposing the complexity of diet?
If you are interested in the interaction between foods and nutrients, an obvious question is how should these be chosen? To date, many studies have not considered specific foods or nutrients but rather freely chosen dietary styles, or diets designed to meet some broad principle. Examples are the Mediterranean and MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet(Reference Morris, Tangney and Wang111), but although there is evidence of a beneficial outcome in terms of cognitive ageing, these data offer little indication of the nature of any nutritional interactions. Are all parts of such diets equally important? What are the optimal and relative amounts of specific nutrients? In fact, are all dietary components necessary?
The Mediterranean diet provides an excellent starting point for studying the interaction between nutrients. One way of dealing with variations in the diet has been to calculate a Mediterranean diet score that indicates the extent to which the diet includes appropriate and inappropriate foods. You gain or lose points for eating specific foods; however, two people can have the same score after consuming very different diets. As such, this tells us little about why the diet was successful. To understand the interaction between nutrients, we need to consider the entire diet rather than a few foods that are believed to be beneficial. It is important to realise that, when you add something to a diet, it is likely that something else will be removed. Similarly, when you remove something from the diet, it may well be replaced with something else. Without dietary monitoring, there is no way of understanding the full picture. The Mediterranean diet is low in refined sugar, cholesterol and trans fats, aspects of the diet that have been associated with poorer cognitive functioning as we age(Reference Van Dyk and Sano112). We must in addition consider the extent to which a positive response to a diet reflects what we do not eat, as well as what is consumed.
To understand the interaction between nutrients, we also need to understand the optimal level of intake and the relative consumption of different nutrients. Although in experimental interventions these can be measured, in most epidemiological surveys little more than a food frequency questionnaire is possible. If so, there should be some indication of portion size. An example of the approach is the examination of fruits and vegetables. When nine cohort studies were reviewed that had monitored cognitive decline or the development of dementia, six found that consuming vegetables, but not fruit, was associated with reduced risk. In three other studies, which compared fruit and vegetables together, a greater intake of vegetables was associated with slower cognitive decline, an association not found with fruit(Reference Loef and Walach113). Chou(Reference Chou, Lee and Chiou114) used a prospective cohort of those over 65 years, measuring over a 2-year period the extent of cognitive decline. The basic quality of the diet was assessed using the Alternative Healthy Eating Index; however, in addition, an index of the variety of vegetables consumed was associated with a positive outcome. Consuming a greater range of vegetables was beneficial, but we still need to understand the relative contribution of different vegetables: do some have a greater effect, or are some ineffective? Starting from dietary styles that are known to be beneficial provides a firm basis on which to build. There are, however, countless thousands of molecules in food items, each potentially interacting in a non-linear fashion with any of thousands of other molecules. By its nature, diet is a complex exposure, potentially making the ‘whole’ very different from the sum of its parts. This raises a challenging question: is it possible or even meaningful to decompose complex dietary patterns into their component parts? Are we barking up the wrong tree?
Current dietary patterns are often based on a priori knowledge of important aspects of diet. For example, the Alternative Healthy Eating Index, which was associated with cognitive decline(Reference Smyth, Dehghan and O’Donnell115,Reference Schulz, Oluwagbemigun and Nothlings116) , has various components: the consumption of vegetables; fruits; nuts and soy proteins; whole grain; deep-fried foods; the ratio of fish to meat and egg; and alcohol. The MIND diet(Reference Morris, Tangney and Wang111) was developed from the DASH diet (Dietary Approaches to Stop Hypertension). It suggests eating whole grain, red wine, nuts, and salad or another vegetable, each day. Berries and poultry are to be eaten twice a week and beans or legumes every other day. Fish should be eaten at least once a week, and cheese, fried food and fast food no more than once per week. Despite their multi-dimensional character, these dietary styles are often condensed using a unidimensional score. Alternatively, linear dimension reduction methods such as principal component analysis, k-means clustering and partial least-squares regression(Reference Schulz, Oluwagbemigun and Nothlings116) are used to simplify the data. However, we may not be able to assume that dietary interactions and associations between diet and disease will be linear. Although more complicated and less easy to interpret, non-linear techniques such as autoencoders, t-distributed stochastic neighbour embedding and manifold learning(Reference Hosseini and Hammer117) could offer a solution, although these are rarely applied to diet.
Machine learning algorithms which can model non-linear and non-additive relations more flexibly could be better placed to capture the richness of nutrition data. For example, using a stochastic gradient boosting regression algorithm applied to detailed dietary, lifestyle, medical, laboratory, anthropometric and microbiota data, Zeevi et al. accurately predicted individual glycaemic responses to food(Reference Zeevi, Korem and Zmora118). In addition, Panaretos et al. found that, compared with linear regression, a random forest algorithm increased the prediction accuracy of nutritional epidemiologic data by 22%(Reference Panaretos, Koloverou and Dimopoulos119). Finally, one study used survival gradient boosted machines and survival random forests to model multiple levels of food classification (e.g., micro- and macro-nutrients) simultaneously to predict cardiovascular disease in the National Health and Nutrition Examination Survey (NHANES). It was reported that this approach was superior to the use of a priori dietary scores (Mediterranean Diet Score, Healthy Eating Index and DASH) which did not improve the performance of the model(Reference Rigdon and Basu120).
The application of machine learning to dietary data is relatively new, and the potential pitfalls, such as atheoretical modelling, large sample requirements and the fact that their relative advantage depends on the importance of non-linearity and interactions, has been reviewed(Reference Morgenstern, Rosella and Costa121). Nonetheless, machine learning has the potential to address some of the challenges inherent in studying the complexity of nutrient–nutrient interactions.
Conclusion
In the light of the above discussion, how should we study the interaction between nutrients? There is a sequence of events. Diet interacts with genetics and host microbiota, with physiological consequences that can either protect or damage the brain. The long-term balance between positive and negative consequences either does or does not lead to brain damage that adversely influences cognitive functioning.
At any one time the impact of the current nutrition is modified by diet over the lifetime. Ideally, we should start at the beginning of this sequence to prevent the onset of a disorder. One approach is to understand the mechanisms that lead to disease and manipulate the diet to modulate these mechanisms. However, given the wide range of risk factors for dementia, it is rare that we will be considering a single mechanism in isolation. Dietary interactions may result from different nutrients influencing different mechanisms in different people. Therefore, a multi-nutrient approach targeting more than one validated risk factor in a specific population could be more efficacious. Although this reductionist approach affords the rigour of traditional scientific methods, we might also need to accept that we are dealing with a complex, integrative and dynamic system:
-
a. Diets include dozens of foods and many thousands of molecules that are likely to interact in complex ways.
-
b. Biological pathways are not necessarily additive or passive; they are dynamic and adaptive and respond in ways that reflect not only their current state, but also their history over seconds, minutes, hours, days and years.
-
c. Inter-individual variations in absorption, metabolism and excretion that reflect differences in lifestyle, genetics, microbiome and demographics will influence the way the system responds to different combinations of nutrients.
-
d. Cognitive test batteries will produce many cognitive measures, with varying degrees of between-subject compensation and various degrees of differentiation between modalities, all of which could be influenced by the various nutrients and molecules in the human diet.
Considered in this way, it is less surprising that recent RCTs have produced unexpected, disappointing and sometimes conflicting results. Is the multi-faceted nature of the effects of dietary interaction on cognitive health such that we may need a holistic approach rather than the traditional reductionist method of dissecting the problem into its component parts and considering one factor in isolation? In this regard, recent advances in high-throughput analyses of metabolites and in machine learning could provide a complementary approach to help us unravel the complex links between diet and human health. It should be noted that ‘big data’ in and of itself will not solve the problem. For example, metabolomics might allow us to measure many thousands of molecules. When analysed from a dynamical systems perspective, that can certainty be informative. However, the tendency is for researchers to ‘home in’ on a one or two molecules which were previously implicated in the human condition. This tells us little about how the system is functioning as an integrated whole.
Although by no means exhaustive, this review highlights six main recommendations to consider when developing and implementing research aimed at investigating the impact of multi-nutrient diet on brain functions. We do not advocate one approach over another (i.e. reductionist versus systems). Both have a place in advancing our understanding of nutrient interactions.
-
1. Establish the working mechanism of individual compounds, and then combinations of compounds.
-
2. Validate the relevance of the mechanisms for the targeted human condition.
-
3. Include current nutrient status, intake or dietary pattern as inclusion/exclusion criteria in the study design.
-
4. Select a participant population that is clinically and biologically appropriate for all nutritional components of the combination.
-
5. Consider a range of cognitive outcomes.
-
6. Consider the limits of reductionism and the ‘gold standard’ randomised controlled trial.
The framework provides the scientific community with a steppingstone for further discussion and refinement. The material in this review serves as a background and guidance document for researchers, companies and organisations that target brain functions, although it is certain to need development and adaptation to ensure the validity and reliability of interventions. It is also worth noting that, whilst we have focused on cognitive ageing, many of the principles discussed here apply equally to multi-nutrient interventions in children, young adults and the middle aged, populations that have garnered increasing interest in recent years. Additionally, as we assume that these principles apply to both clinical conditions and ‘normal age-related decline’, at this point, we did not differentiate between the two. Hopefully, as the field develops, these guiding principles will help to identify interactions between aspects of diet and provide an evidence base for developing new interventions to facilitate cognition and/or delay cognitive decline.
Acknowledgements
The research question addressed in this publication and potential contributing experts in the field were identified by the Nutrition and Brain Health Task Force. Members of this task force are listed on the ILSI Europe website. Once the Expert Group (EG) was formed, the research project was handed over to them to independently refine the research question. Consequently, the EG carried out the work, that is, collecting/analysing data/information and writing the scientific paper, independently of other activities of the task force. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a pre-competitive setting for public–private partnership. ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +3227710014. The opinions expressed herein, and the conclusions of this publication, are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies, nor any regulatory authority.
This work was conducted by an EG of the European branch of the International Life Sciences Institute, ILSI Europe. According to ILSI Europe policies, the EG is composed of at least 50% external non-industry members. The complete composition of the EG can be found on the ILSI Europe website. Experts are not paid for the time spent on this work; however, the non-industry members within the expert group were offered support for travel and accommodation costs from the Nutrition and Brain Health Task Force to attend meetings to discuss the manuscript and a small compensatory sum (honorarium) with the option to decline.
L.G., H.Y., N.K., D.B., L.B., A.J.K. and D.V. declare that they have no competing interests. J.F., A.P., L.T. and J.S. are employees of industry, as declared under affiliation.
All authors contributed to the conception, design, writing and revision of the work.
L.G. and D.V. contributed to overall management and revision of the work.