Take-Home Points
The therapeutic actions of traditional antidepressants are often delayed by weeks and are associated with significant side effects.
The use of agents with more rapid onsets of action, such as ketamine, is restricted due to the need for monitoring, risk of serious side effects, and the potential for abuse/misuse.
Dextromethorphan has similar pharmacological properties to ketamine and is primarily thought to act through a combination of activities at the N-methyl-D-aspartate (NMDA) receptor, serotonin transporter (SERT), norepinephrine transporter (NET), and sigma-1 receptor. When combined with bupropion, a well-established antidepressant, there may be a synergistic therapeutic effect. Bupropion primarily acts through NET and dopamine transporter (DAT) with additional cytochrome P450 2D6 (CYP450 2D6) inhibition.
In contrast to ketamine, dextromethorphan/bupropion is orally administered and does not appear to induce dissociative effects at therapeutically active concentrations.
Introduction
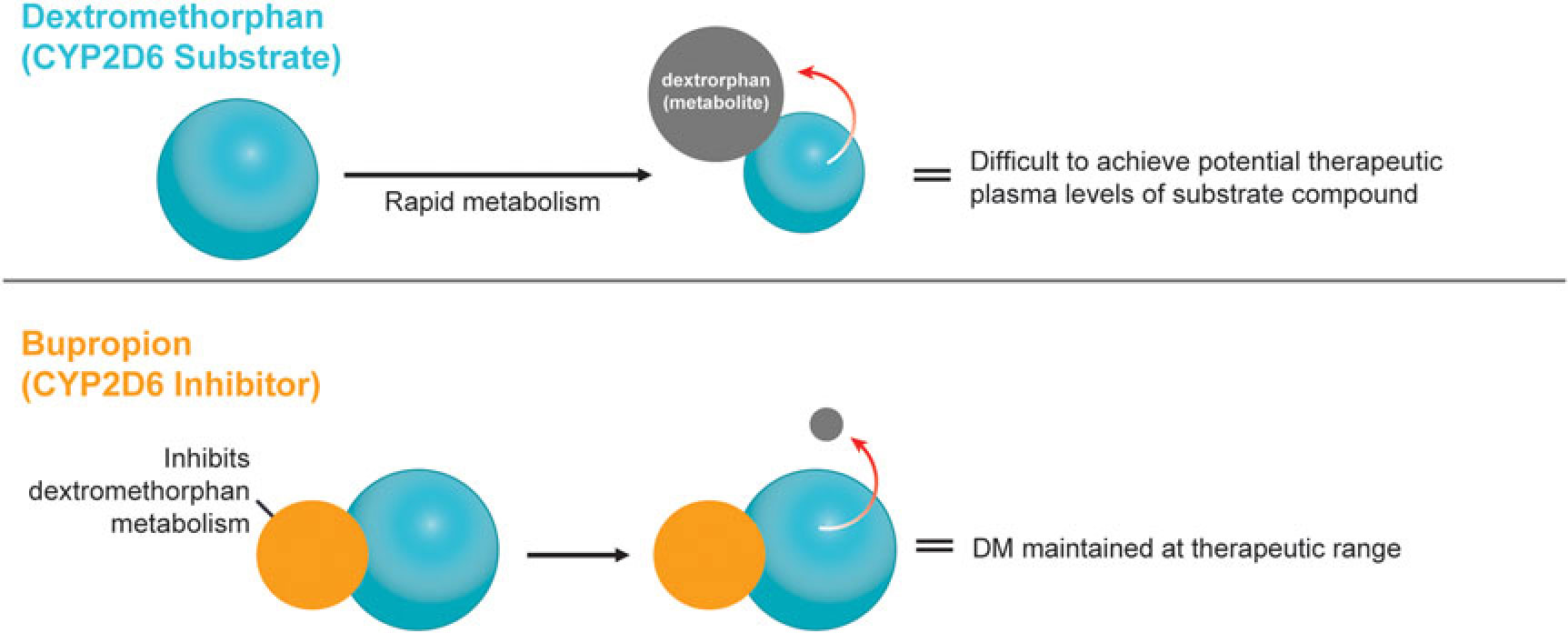
A novel, oral, investigational NMDA (N-methyl-d-aspartate) receptor antagonist with multimodal activity is currently being developed for the treatment of central nervous system disorders. This agent combines the NMDA antagonist dextromethorphan with the norepinephrine–dopamine reuptake inhibitor bupropion.Reference Stahl1 Dextromethorphan is rapidly metabolized by cytochrome P450 2D6 (CYP2D6), making it difficult to achieve therapeutic plasma levels following oral administration.2 Bupropion and its metabolites are CYP2D6 inhibitors3 and the administration of bupropion in combination with dextromethorphan leads to significant increases in dextromethorphan exposure at all evaluated doses in three Phase I studies (Figure 1).Reference O’Gorman, Iosifescu and Jones4 Bupropion, with its distinct centrally-acting mechanisms of action, is a compelling choice as metabolic inhibitor of dextromethorphan and suggests the potential for pharmacological synergy and clinical use across a broad range of neuropsychiatric conditions. Dextromethorphan/bupropion is currently in late-stage clinical development for major depressive disorder (including treatment-resistant depression), Alzheimer’s disease agitation, and smoking cessation.Reference O’Gorman, Iosifescu and Jones4
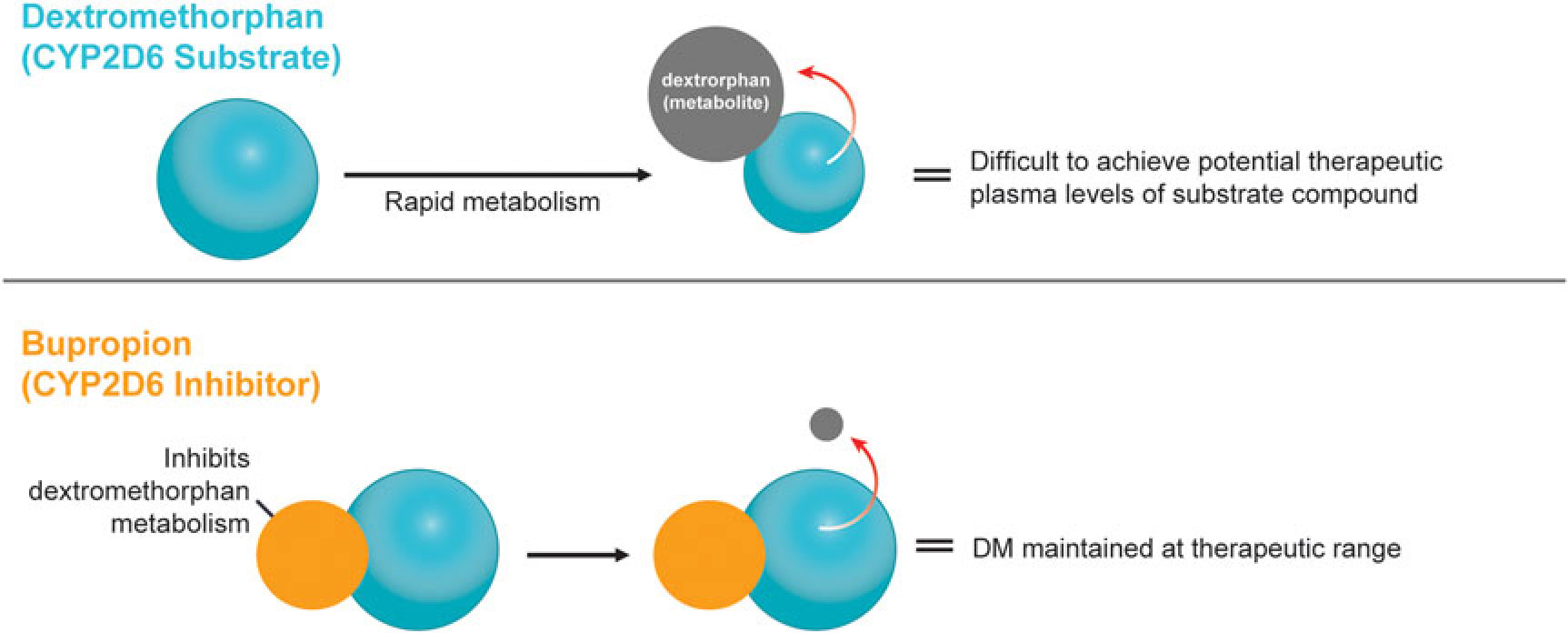
Figure 1 Dextromethorphan is rapidly metabolized by CYP2D6 following oral administration in humans. Co-administration with a CYP2D6 inhibitor, such as bupropion, inhibits dextromethorphan metabolism, maintaining dextromethorphan concentrations in the therapeutic range.
Beyond Monoamines: Unconventional Antidepressant Mechanisms and Multimodal Therapeutic Approaches
The classic monoamine hypothesis of depression posits in an oversimplified manner that depression arises due to a deficiency in monoamine neurotransmitters. Although direct evidence for the monoamine hypothesis is lacking, virtually all currently available antidepressants directly affect one or more monoamine neurotransmitter systems. However, despite the fact that such agents increase monoamine levels shortly after initiating treatment, the therapeutic benefits are often delayed by several weeks.Reference Stahl1
As a result, the focus has now shifted away from the neurotransmitters themselves to their receptors and the downstream molecular events that these receptors trigger in order to explain the mechanism of action of antidepressants. For example, antidepressant-mediated alterations in neurogenesis and neuroplasticity have drawn increased attention. Treatment with agents targeting the monoamine pathway, such as selective serotonin reuptake inhibitors (SSRIs), has been shown to increase neurogenesis in the dentate gyrus of the hippocampus.Reference Crupi, Marino and Cuzzocrea5 These effects may be mediated by activating cAMP (cyclic adenosine monophosphate) response element binding protein (CREB), which can elicit the expression of numerous genes involved in neuroplasticity, including brain-derived neurotrophic factor (BDNF).Reference Stahl1 Clinical studies have indicated that chronic antidepressant treatment can restore abnormally low BDNF levels and such restoration has been correlated with reduced scores on depression rating scales.Reference Sen, Duman and Sanacora6
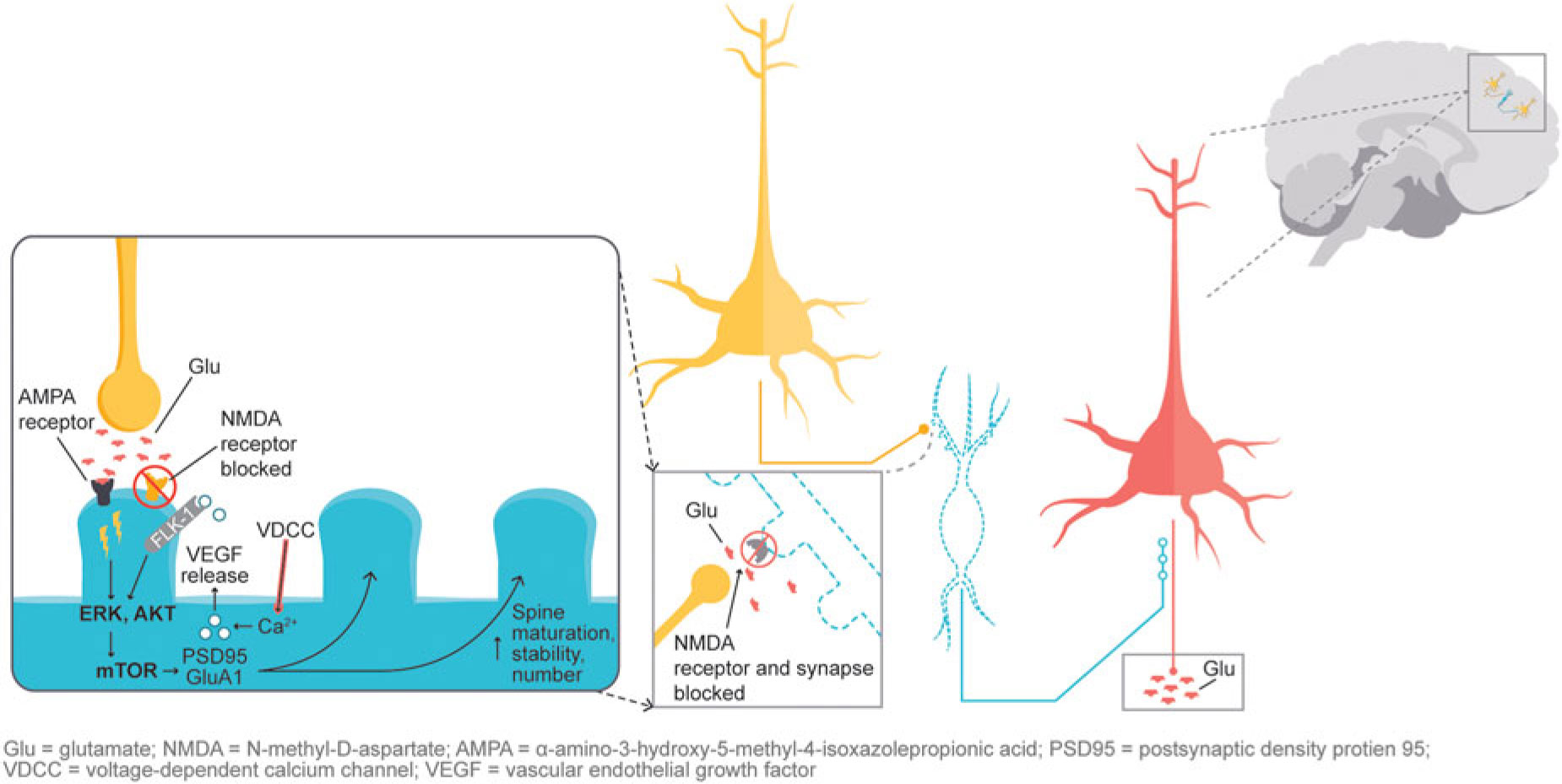
In addition, glutamatergic signaling is thought to play a role in maintaining neuroplasticity. Glutamate-mediated activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors leads to activation of the extracellular signal-regulated kinase/protein kinase B, or ERK/AKT signal transduction cascade, triggering the mammalian target of rapamycin (mTOR) pathway. Activation of the mTOR pathway has been reported to increase synaptic protein expression, resulting in increased dendritic spine density.Reference Stahl1,Reference Deyama, Bang and Wohleb7 Synaptic and intracellular events stimulated by ketamine suggest that NMDA antagonism produces a “glutamate surge” that is then hypothesized to increase AMPA/NMDA receptor flux, stimulating intracellular signaling cascades (Figure 2). Studies have shown that both ketamine and dextromethorphan require activation of AMPA receptors to mediate their antidepressant effects.Reference Stahl1,Reference Niciu, Ionescu, Richards and Zarate8,Reference Freudenberg, Celikel and Reif9,Reference Zarate, Machado-Vieira and Henter10,Reference Nguyen and Matsumoto11
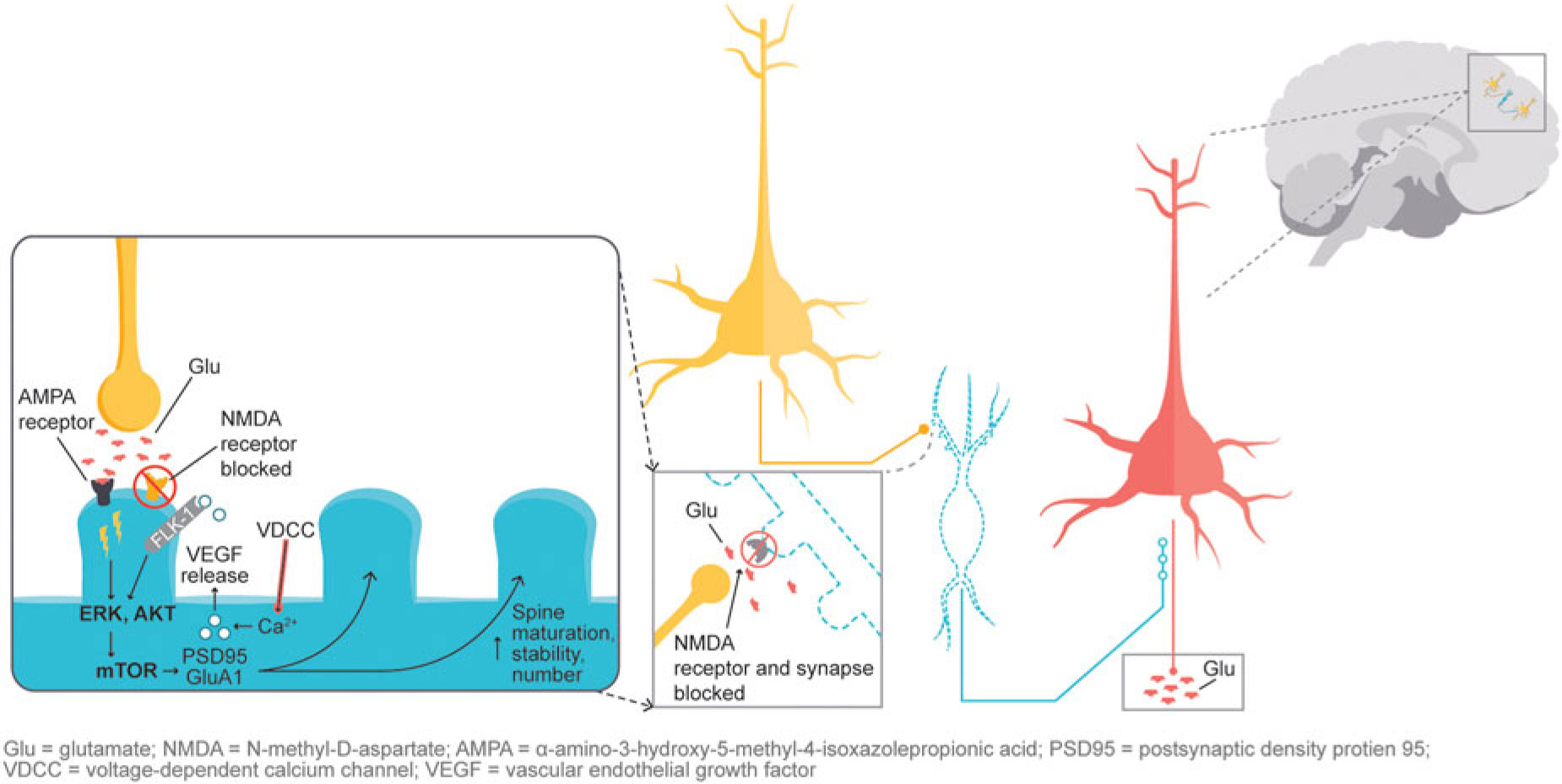
Figure 2 NMDA blockade-mediated activation of AMPA receptors induces downstream cascades involved in neural plasticity that are hypothesized to underlie antidepressant effects.
Dextromethorphan/bupropion combines the mechanisms of action of several distinct antidepressant therapeutic classes into one therapeutic agent. Both dextromethorphan and bupropion increase norepinephrine availability by inhibiting reuptake and acting as alpha-4-beta-2 nicotinic (nACh) antagonists. In addition, bupropion increases dopamine availability by blocking reuptake, while dextromethorphan increases glutamate by acting as an NMDA receptor antagonist and serotonin (by both acting as a serotonin reuptake inhibitor and boosting serotonin activity in the dorsal raphe via sigma-1 agonism) (Figure 3).Reference Stahl1

Figure 3 Dextromethorphan (Panel A) is an NMDA receptor antagonist that acts as a SERT and NET inhibitor, nACh α4β4 antagonist, sigma-1 and mu opioid receptor agonist. Bupropion (Panel B) is a CYP2D6 inhibitor that acts as a NET and DAT inhibitor and a nACh α4β4 antagonist.
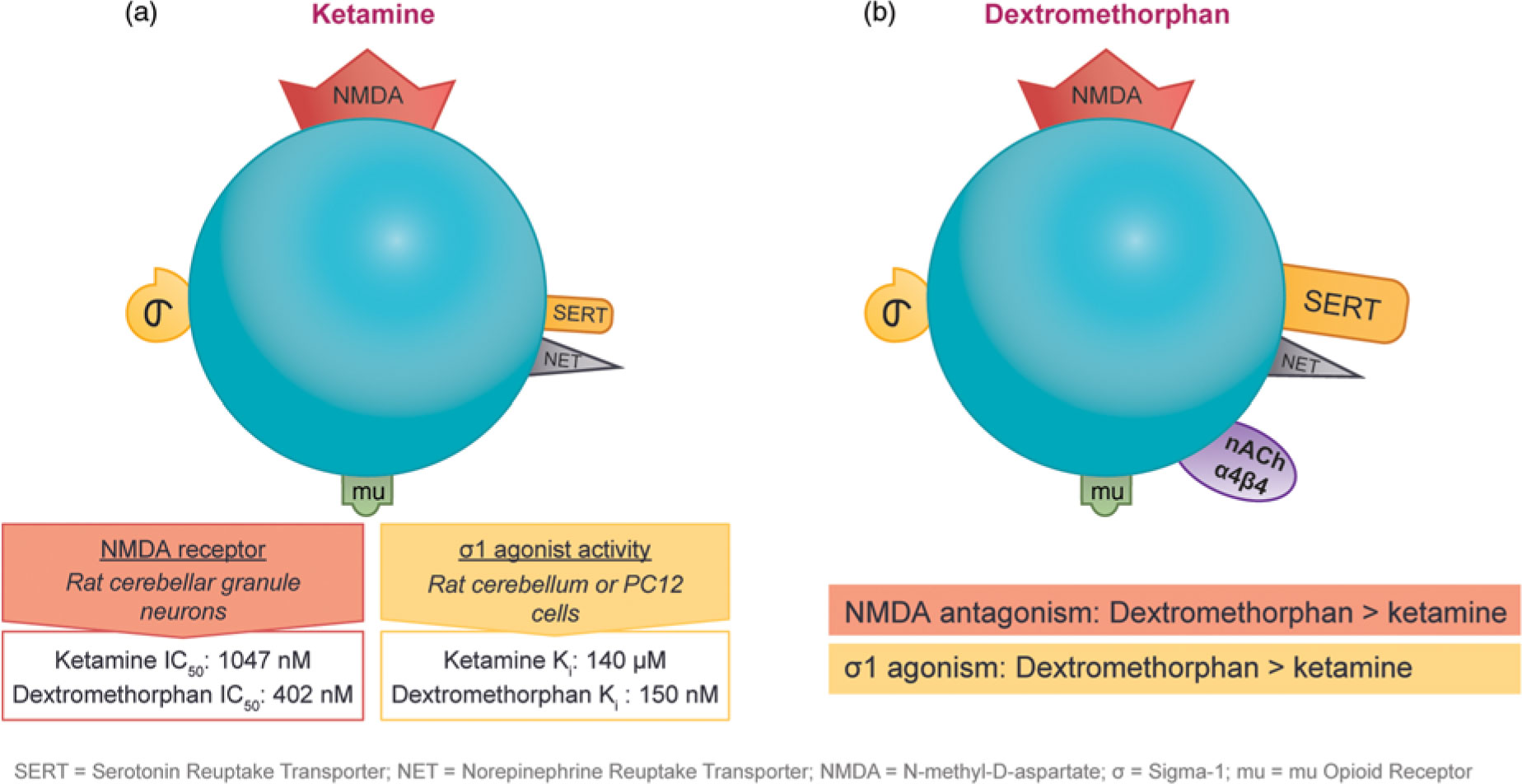
The observation that subanesthetic doses of ketamine induce immediate antidepressant effects in patients with treatment-resistant unipolar or bipolar depression has spurred the search for agents with similar pharmaceutical properties. Ketamine and dextromethorphan display activity at the same receptors; however, dextromethorphan exhibits both higher affinity as an NMDA receptor antagonist and greater potency at the sigma-1 site than ketamine in some cellular assay systems (Figure 4).Reference Stahl1,Reference Berman and Murray12,Reference Werling, Keller, Frank and Nuwayhid13,Reference Robson, Elliott, Seminerio and Matsumoto14,Reference Lauterbach15
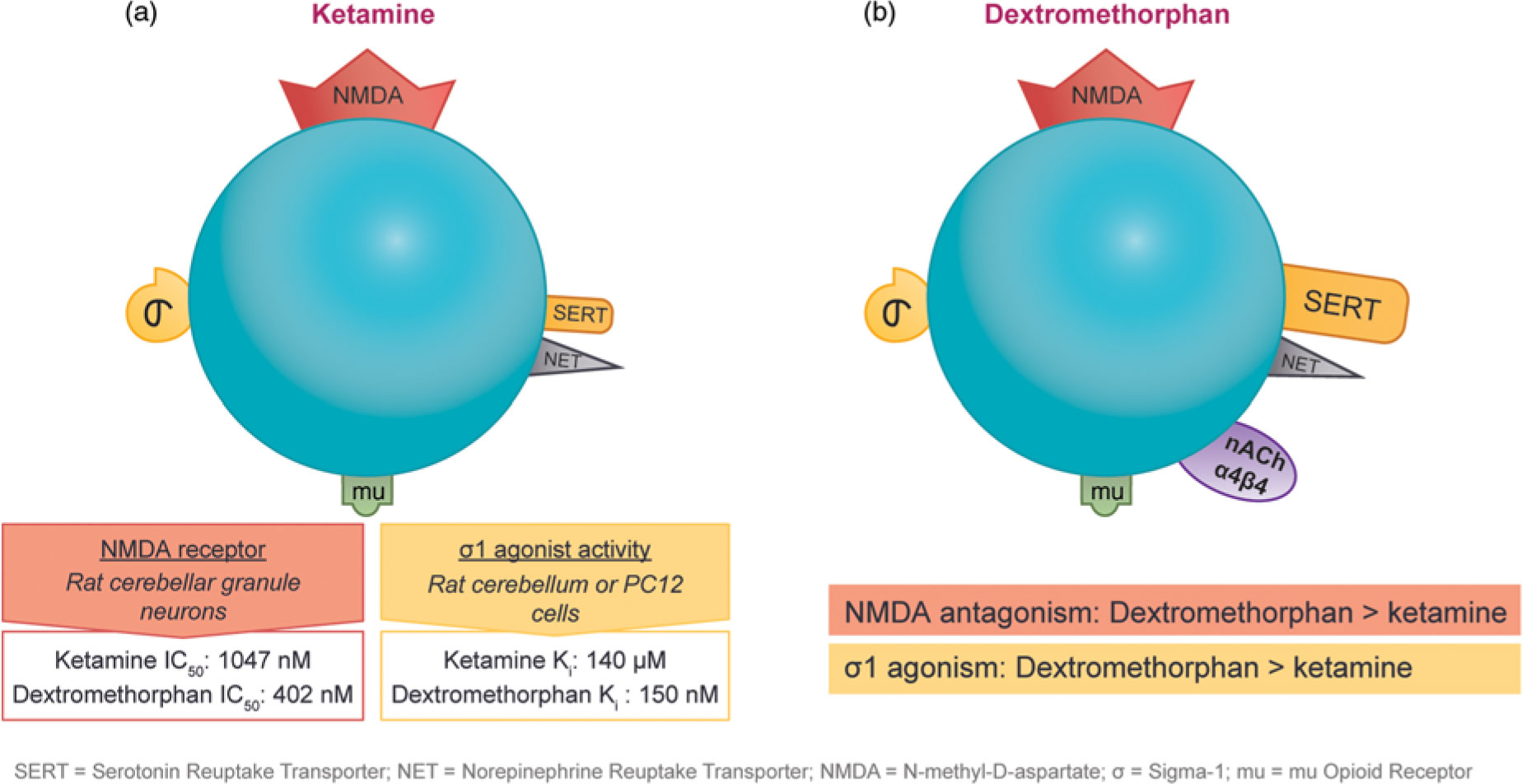
Figure 4 The pharmacological properties of ketamine and dextromethorphan share significant overlap. Although both agents have affinity for the NMDA receptor, the sigma-1 potency and NMDA receptor affinity of dextromethorphan are higher than those of ketamine in some cellular assay systems.
Interestingly, opioid receptor antagonism via administration of naltrexone prior to the intravenous administration of ketamine attenuated the antidepressant effects (but not the dissociative effects) of ketamine in adults with treatment-resistant depression, suggesting that the acute antidepressant effects of ketamine require opioid activation but the dissociative effects do not.Reference Williams, Heiferts and Blasey16 One explanation for this observation is that crosstalk between opioid receptor-mediated signaling and NMDA receptor-mediated signaling interferes with ketamine’s ability to activate mTOR.Reference Wang and Kaplin17 However, treatment of patients with the mTOR inhibitor rapamycin prior to ketamine infusion potentiated rather than blocked the antidepressant effects of ketamine.Reference Heiferts, Williams and Blasey18 Therefore, further research is required to fully elucidate the role of opioid signaling in the antidepressant effects of ketamine. Based on several studies examining the effect of naloxone, an opioid receptor antagonist, mu opioid receptor activity has not been shown to be clinically relevant to dextromethorphan, however the antidepressant effect has not been examined.Reference Gaginella, Bertko and Kachur19,Reference Kachur, Morgan and Gaginella20
Considerations for Major Depressive Disorder and Treatment-Resistant Depression
Major depressive disorder continues to be a leading cause of disease burden, both in the United States and globally. In 2017, an estimated 17.3 million adults over the age of 18 in the United States experienced at least 1 major depressive episode, while over 300 million individuals worldwide are estimated to suffer from depression.21,22 Studies indicate that the majority of patients inadequately respond to monoamine-targeting therapy. Approximately 63% of patients failed to achieve remission after initial treatment with an SSRI.Reference Rush, Trivedi and Wisniewski23 And of those patients who failed to remit, 69% failed to achieve remission upon switching to a second antidepressant.Reference Rush, Trivedi and Wisniewski23 In patients who do achieve remission, residual symptoms, including insomnia, weight gain, and impaired concentration/decision making, are common.Reference Nierenberg, Husain and Trivedi24,Reference McClintock, Husain and Wisniewski25
At present, only a few treatment options have been FDA-approved for treatment-resistant depression or as an adjunct to antidepressants for major depressive disorder: SYMBYAX® (olanzapine in combination with fluoxetine), SPRAVATO™ (esketamine), ABILIFY® (aripiprazole), and REXULTI® (brexpiprazole).26,27,28,29 However, the use of SPRAVATO™ in patients with treatment-resistant depression has been restricted to approved healthcare settings due to the risks of sedation, dissociation, and abuse/misuse, highlighting the need for more easily accessible agents with fewer side effects.27
Research suggests that combination therapy with multi-modal agents is more effective than antidepressant monotherapy. While 25% of patients treated with an SSRI alone for 6 weeks achieved remission, 52% of those treated with an SSRI in combination with a noradrenergic and specific serotonergic antidepressant (NaSSA) and 46% of those treated with an NaSSA in combination with bupropion achieved remission.Reference Blier, Ward and Tremblay30
The multimodal pharmacological activity of dextromethorphan/bupropion therapy also contributes to more rapid efficacy than antidepressant monotherapy. In the Phase 2, multicenter, randomized, double-blind, active-controlled ASCEND (Assessing Clinical Episodes in Depression) trial, 80 patients with a confirmed diagnosis of moderate to severe major depressive disorder were randomized in a 1:1 ratio to receive 45 mg dextromethorphan/105 mg bupropion (n=43) or bupropion (105 mg) (n=37) twice daily for 6 weeks. Treatment with dextromethorphan/bupropion resulted in significantly lower Montgomery-Åsberg Depression Rating Scale (MADRS) total scores by week 2 as compared to bupropion monotherapy. In addition, 26% of patients treated with dextromethorphan/bupropion had achieved remission by week 2 versus 3% of those receiving bupropion monotherapy. Notably, no NMDA dissociative/psychotomimetic events were observed in the ASCEND trial.Reference Anderson, Iosifescu and Jacobson31
Summary and Conclusions
Dextromethorphan/bupropion is an orally administered, rapidly-acting, investigational NMDA receptor antagonist with multimodal activity. The formulation achieves pharmacologic synergy by simultaneously targeting monoamines, NMDA receptors, and sigma-1 receptors, resulting in more rapid and robust decreases in depression rating scale scores than bupropion treatment alone. With its similar pharmacological properties to ketamine, dextromethorphan/bupropion represents a promising new investigational agent for depression.
Disclosure information
Stephen M. Stahl, M.D., PhD, Dsc (Hon.) is an Adjunct Professor of Psychiatry at the University of California San Diego, Honorary Visiting Senior Fellow at the University of Cambridge, UK and Director of Psychopharmacology for California Department of State Hospitals. Over the past 36 months (January 2016 - December 2018) Dr. Stahl has served as a consultant to Acadia, Adamas, Alkermes, Allergan, Arbor Pharmaceutcials, AstraZeneca, Avanir, Axovant, Axsome, Biogen, Biomarin, Biopharma, Celgene, Concert, ClearView, DepoMed, Dey, EnVivo, EMD Serono, Ferring, Forest, Forum, Genomind, Innovative Science Solutions, Intra-Cellular Therapies, Janssen, Jazz, Lilly, Lundbeck, Merck, Neos, Novartis, Noveida, Orexigen, Otsuka, PamLabs, Perrigo, Pfizer, Pierre Fabre, Reviva, Servier, Shire, Sprout, Sunovion, Taisho, Takeda, Taliaz, Teva, Tonix, Trius, Vanda, Vertex and Viforpharma; he has been a board member of RCT Logic and Genomind; he has served on speakers bureaus for Acadia, Astra Zeneca, Dey Pharma, EnVivo, Eli Lilly, Forum, Genentech, Janssen, Lundbeck, Merck, Otsuka, PamLabs, Pfizer Israel, Servier, Sunovion and Takeda and he has received research and/or grant support from Acadia, Alkermes, AssureX, Astra Zeneca, Arbor Pharmaceuticals, Avanir, Axovant, Biogen, Braeburn Pharmaceuticals, BristolMyer Squibb, Celgene, CeNeRx, Cephalon, Dey, Eli Lilly, EnVivo, Forest, Forum, GenOmind, Glaxo Smith Kline, Intra-Cellular Therapies, ISSWSH, Janssen, JayMac, Jazz, Lundbeck, Merck, Mylan, Neurocrine, Neuronetics, Novartis, Otsuka, PamLabs, Pfizer, Reviva, Roche, Sepracor, Servier, Shire, Sprout, Sunovion, TMS NeuroHealth Centers, Takeda, Teva, Tonix, Vanda, Valeant and Wyeth.