INTRODUCTION
Measles is still a major childhood disease, which was associated with an estimated 614 000 fatal cases in 2002 [1]. The introduction of live attenuated measles vaccines has largely abrogated endemic transmission of measles virus (MV) in the industrialized world, but vaccination has been less successful in developing countries. This is thought to be the combined result of insufficient vaccination coverage and inherent disadvantages of the live attenuated vaccine such as the need for cold chain maintenance and interference by maternal antibodies [Reference Stittelaar, De Swart and Osterhaus2, Reference Putz, Bouche, De Swart and Muller3]. The World Health Organization (WHO), UNICEF and other partners developed a Global Measles Strategic Plan for the period 2001–2005 aimed at global mortality reduction and regional elimination, after which period the feasibility and desirability of global eradication of measles virus will be evaluated [4].
Measles vaccination is not effective in very young infants, mainly due to interference with MV-specific maternal antibodies and immaturity of the immune system [Reference Albrecht, Ennis, Saltzman and Krugman5, Reference Gans, Yasukawa and Rinki6]. In most industrialized countries with low measles incidence the first measles vaccination is, therefore, carried out between the ages of 12 and 15 months. However, in countries with higher measles incidence this strategy results in high morbidity and mortality in infants below that age, due to the resulting ‘window of susceptibility’: a large number of infants will have lost their MV-specific maternal antibodies several months before their first vaccination. Therefore, in developing countries measles vaccination is usually provided at the age of 9 months.
Sudan is one of the member states of the Eastern Mediterranean Region of the WHO, which has a measles elimination target of 2010. However, measles vaccination coverage is currently insufficient to prevent endemic transmission of wild-type measles viruses, resulting in substantial measles-related morbidity and mortality [Reference Ibrahim, Mustafa and Mukhtar7–Reference El Mubarak, Yuksel, Mustafa, Ibrahim, Osterhaus and De Swart9]. In 2001 4362 measles cases were reported (incidence 12/100 000), but this is thought to be an underestimation of the true numbers [Reference Gaafar, Moshni and Lievano8]. According to the current Expanded Programme of Immunization (EPI) in Sudan, infants receive one measles vaccination at the age of 9 months, without a second dose at a later age.
For monitoring the effectiveness of measles control programmes, periodic seroepidemiological studies are essential. A previous study on the placental transfer and decay of MV-specific maternal antibodies in Nigerian infants showed that at the age of 4 months only 17% of the infants still had protective antibody titres, strongly suggesting the need for alternative vaccination strategies [Reference Hartter, Oyedele, Dietz, Kreis, Hoffman and Muller10]. In the present paper, we report the results of a seroepidemiological study based on filter-paper blood samples.
Collection and storage of serum samples is often difficult to organize, especially in developing countries. As an alternative, dried blood spots collected on filter paper have been used since the 1960s [Reference Brody, McAlister, Haseley and Lee11]. After reconstitution antibodies can be detected in a similar way as in serum, which has been validated for several different assay systems by testing paired serum and filter-paper blood samples [Reference Condorelli, Scalia and Stivala12–Reference Riddell, Byrnes, Leydon and Kelly16]. Disadvantage of filter-paper blood samples is that the quantitative aspect of the results is less well controlled, since the original volume of blood that is reconstituted is always an estimate. In addition, reconstituted samples are often toxic for cells, which makes these samples unsuitable for virus neutralization assays. However, these disadvantages are compensated for by the relative ease of blood collection (by heel- or finger-prick), sample storage and sample transport, which makes seroepidemiological studies practical and affordable in remote tropical areas with limited infrastructure.
The objectives of the present community-based prospective cohort study were to (a) assess levels of maternally derived MV-specific antibodies at birth, (b) monitor waning of these antibodies and (c) measure newly acquired MV-specific antibodies at different time points during infancy.
METHODS
Study area
The study area included 14 small villages ∼40 km southeast of Khartoum city, in a rural area with an estimated total population of 300 000. An infrastructure for the collection of clinical and epidemiological data in these villages was previously established in the framework of several community-based antenatal/perinatal projects that had been going on since 1985 [Reference Ibrahim, Omer, Amin, Babiker and Rushwan17, Reference Ibrahim, Babiker, Amin, Omer and Rushwan18]. The projects were based on upgrading the skills of the village midwife (who may be the only health provider available) in the rural areas to monitor pregnancies, detect and refer early at-risk pregnancies, and report on infant births and deaths. Vaccinations were provided by mobile EPI teams.
Study design
The birth cohort study included all babies born between March 1997 and June 1998. Consenting mothers were interviewed shortly after birth and a typed questionnaire containing the mothers' medical, obstetrical and social history was completed. The village midwife collected cord blood samples on filter paper (Whatman no. 3), which were air dried and stored at room temperature. During weekly follow-up visits the filter papers were transported to the laboratory and subsequently stored at −20°C. Heel-prick filter-paper blood samples were collected from the infants at ages 6, 12 and 24 months, and additional information including natural measles infection and age at measles vaccination of the infant was obtained (in the majority of cases by reporting, not by vaccination card). The study was approved by the Medical Ethical Committee of the University of Khartoum.
Cohort characteristics
A total of 205 questionnaires were collected between March 1997 and June 1998. The mothers (median age 25, range 14–45 years) had a median number of three (range 0–11) previous live births. The majority (88%) of the mothers reported to have had measles as a child; none had been vaccinated against measles or had measles during pregnancy. All infants were born at home: 106 males and 100 females (including one twin-birth), all except one had a gestational age >37 weeks. The mean birth weight was 2880 g (range 1500–3000 g), 17 (8%) babies weighed <2500 g. A total of 196 cord blood samples were available for analysis (the remaining 10 samples were lost).
Of the 196 infants sampled by cord blood, 172 (88%) could be sampled by heel-prick at the age of 6 months. Thirteen (6·6%) of the infants had died on days 1, 2, 4, 12, or months 2, 2½, 3, 4, 5 (n=3) or 5½ (n=2) after birth. Seven infants had moved out of the area, and four refused. In addition to these 172 infants, the 10 infants of whom no cord blood sample was available were also sampled at the age of 6 months, bringing the total number of 6-month samples to 182. Of the 182 infants sampled at the age of 6 months, 172 could be sampled again at the age of 12 months. Five additional infants had died [aged 8 (n=2), 9 (n=2) and 10 (n=1) months), giving a total infant mortality, i.e. death in the first year of 91/1000 live births. One more infant had moved outside the area and four additional infants refused blood sampling. At age 24 months, 166 (97%) out of the 172 infants were re-sampled. Two more infants had died (aged 13 and 23 months) bringing the total deaths to 20, and one moved out of the area (total moved=9); three more refused blood sampling (total refusals=11).
Sample processing
For serological analyses, the dried blood spots were reconstituted in phosphate buffered saline (PBS) supplemented with 2% foetal bovine serum (FBS). A circle with an approximate diameter of 1 cm was incubated in 1 ml PBS/2% FBS overnight at 4°C on a rotating device. It was estimated that this filter paper contained approximately one drop (25 μl) of blood, i.e. between 10 and 15 μl of serum. Therefore, the supernatant of the reconstituted filter paper blood sample was treated as an approximate 1:100 serum dilution. A quality control was introduced by measuring the total IgG1 level in the reconstituted sample using a commercial kit (CLB, Amsterdam, The Netherlands): if this value was below the normal range for human serum (below 2·4 g/l for cord blood and below 2·0 and 2·9 g/l for blood of infants aged 6–12 and 18–24 months respectively, according to the manufacturer's instructions), the results of the other serological assays were rejected.
ELISA
Whole MV-specific and MV nucleoprotein (N)-specific IgG levels were measured in in-house developed indirect ELISA assays [Reference El Mubarak, Ibrahim and Vos19]. Briefly, medium-binding microtitre plates (Greiner Labor Technik, Nürtingen, Germany) were coated with a β-propiolactone-inactivated whole measles preparation or with a baculovirus-expressed recombinant N (a kind gift of Dr T. F. Wild) in PBS at previously established optimal concentrations. After washing the plates, the reconstituted filter paper samples were diluted 1:3 in ELISA buffer (Meddens Diagnostics, Vorden, The Netherlands) supplemented with 5% normal goat serum, reaching an estimated final dilution of 1:300. After 1 h incubation at 37°C, plates were washed, incubated with a peroxidase-labelled goat-anti-human IgG conjugate [F(ab′)2; BioSource International, Camarillo, CA, USA], and subsequently developed using tetramethyl-benzidine as substrate. All filter paper samples were tested in duplicate. Each plate included a titration curve of the 2nd International Standard for Measles [in duplicate, serum 66/202, NIBSC, Hertfordshire, UK, 5 international units (IU) per ml], the resulting extinctions at 450 nm in five different plates are shown in Figure 1 (means±standard deviations). Per individual ELISA plate the results of the titration curve were used to express the results of the tested samples in IU/ml.
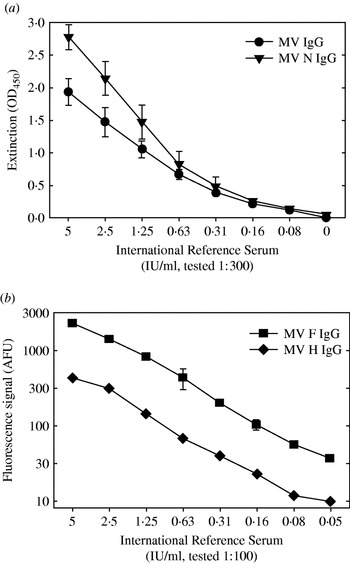
Fig. 1. Results of the four different serological assays used when testing a serial dilution of the 2nd International Standard for Measles [serum 66/202, NIBSC, Hertfordshire, UK, 5 international units (IU) per ml]. (a) ELISA plates coated with an inactivated whole measles virus preparation (–•–) or a recombinant-baculovirus produced nucleoprotein (–▾–) were incubated with the serial dilution of the reference serum at a further dilution of 1:300, similar to the test samples in these assays. After washing the plates were incubated with a peroxidase-labelled anti-human IgG conjugate. Results are shown as the extinction measured at 450 nm (mean±standard deviation of five measurements). (b) F- or H-expressing transfected human melanoma cells were incubated with a serial dilution of the reference serum at a further dilution of 1:100, similar to the test samples in these assays. After washing the cells were incubated with a FITC-labelled anti-human IgG conjugate, and immunofluorescence was quantified in a FACS. Results are shown as fluorescence signal in arbitrary fluorescence units (mean±standard deviation of triplicate measurements).
Immunofluorescence
MV fusion protein (F) and haemagglutinin (H)-specific IgG levels were detected in a FACS-measured immunofluorescence assay using transfected human melanoma cell lines as targets, as described previously [Reference De Swart, Vos, UytdeHaag, Osterhaus and Van Binnendijk20]. Briefly, melanoma cells expressing either F (Mel-JuSo/MV-F) or H (Mel-JuSo/MV-H), or the untransfected parental cell line (Mel-JuSo/wt), were incubated with reconstituted filter paper samples. After 1 h on ice the cells were washed and stained with FITC-labelled rabbit anti-human IgG [F(ab′)2 fragments; Dako, Glostrup, Denmark]. Results are expressed as the fluorescence signal (histogram peak channel) measured on a FACScan (Becton-Dickinson, Mountain View, CA, USA), in arbitrary fluorescence units (AFU). This fluorescence intensity was previously shown to correlate with the quantity of specific antibodies [Reference De Swart, Vos, UytdeHaag, Osterhaus and Van Binnendijk20]. An arbitrary cut-off a signal of 30 AFU was used as previously defined [Reference De Swart, Vos, UytdeHaag, Osterhaus and Van Binnendijk20], sensitivity and specificity of these assays was assessed in an earlier study [Reference Hartter, De Swart and Hanses21]. The relationship between fluorescence signals and international units is shown in Figure 1. Twelve (6%) of the reconstituted cord blood samples showed non-specific fluorescence on Mel-JuSo/wt cells, and were discarded from further analysis. In the other samples background signals measured on Mel-JuSo/wt cells were consistently below 15 (not shown).
RESULTS
Baseline data and sample quality
Measles vaccination coverage during the first year of life was reported to be 121/172 (70%), whereas at the age of 24 months 131/166 (79%) were reported to be vaccinated. Nine infants developed clinical measles during the study period, at ages 9 (n=1), 12 (n=2), 13 (n=1) and 18 (n=5) months. All except one were reported to have been vaccinated against measles; no samples were collected for laboratory confirmation of the infection.
The median IgG1 concentration in the reconstituted cord blood samples was 12·6 g/l (range undetectable–28·8, n=9 samples excluded from further analysis); while in heel-prick samples collected at ages 6, 12 and 24 months this was 8·0 (range 2·15–19·6), 9·0 (range 2·4–24·2) and 9·5 (range 4·6–12·9) g/l respectively (no further samples excluded from analysis based on total IgG1 levels).
MV serology
As shown in Figure 2, MV-specific IgG levels in cord blood samples were usually high: in the majority of samples ELISA IgG antibody levels were above the detection limit of 5 IU/ml. Based on the ELISA assays only 2–5% of the samples contained <0·2 IU/ml, while in the immunofluorescence assays 9 and 23% of the samples were below the arbitrary cut-off for F- and H-specific IgG antibodies respectively.

Fig. 2. Specific IgG levels in cord blood samples (left two panels) or heel-prick samples prospectively collected at ages 6, 12 or 24 months (right six panels). Samples were reconstituted from dried blood spots collected on filter paper, and tested in MV-specific or N-specific ELISA (upper panels, results expressed in IU/ml) or in F- or H-specific FACS-measured immunofluorescence assays (lower panels, results expressed in arbitrary fluorescence units, AFU). In the upper panels the dashed lines indicate antibody levels associated with protection from disease (0·2 IU/ml), in the lower panels they indicate a previously established arbitrary cut-off level (30 AFU). Symbols are shown in black if subjects were vaccinated ⩾1 month prior to sample collection; triangle-shaped symbols are used for subjects who were diagnosed with clinical measles ⩾1 month prior to sample collection. Numbers in the corners of the panels indicate the percentage samples in the respective quadrant.
At the age of 6 months the levels of maternal antibodies had dropped substantially. In ELISA 59–62% had specific IgG levels <0·2 IU/ml, while F- and H-specific IgG antibodies were below the cut-off in 84 and 92% of the samples respectively.
At ages 12 and 24 months the specific IgG levels were no longer normally distributed, but two clusters became apparent: one with low and one with high antibody levels. These clusters were discernible both by ELISA and immunofluorescence (Figure 2, upper and lower panels respectively), and the results of the different methods correlated well (data not shown). It was concluded that the cluster of samples with the higher antibody levels represented subjects with acquired MV-specific antibodies. At the age of 12 months these were detected in 45–58% of the population depending on the assay, while at 24 months this was 65–85% of the population.
Before the 12-month sampling 115/172 (67%) of the infants were reported to be vaccinated against measles. Vaccinations had been provided at the following ages: 7 months (n=4), 9 months (n=104), 10 months (n=4) or 11 months (n=3). Vaccinations provided at the age of 12 months were excluded, since seroconversion could not reliably be measured so early after vaccination. Of these only 58% had seroconverted at age 12 months. Prior to the 24-month sampling another 16 infants had been vaccinated at ages 12 months (n=6), 13 months (n=1), 15 months (n=1), 16 months (n=1), 18 months (n=5) or 24 months (n=2), bringing the total vaccination coverage to 131/166 (79%). Of these 131 infants 97 (74%) had seroconverted at the age of 24 months.
DISCUSSION
We have used dried blood spots collected on filter paper to study the kinetics of maternally derived and acquired MV-specific antibodies during early childhood in rural Sudan. At 6 months of age, maternal antibodies had waned below cut-off values in approximately half of the infants. At ages 12 and 24 months newly acquired MV-specific antibodies could be detected. Measles vaccination had been provided to 67 and 79% of the infants during their first and second year of life respectively. However, of these vaccinated infants only 74% had seroconverted by the age of 24 months. This study demonstrates the usefulness of filter-paper blood samples for prospective and cross-sectional seroepidemiological studies.
At age 6 months approximately half of the cohort still had detectable MV-specific antibodies, while at age 12 months maternal antibodies had largely waned, suggesting that 9 months is an appropriate age for vaccination in this area. In a study in rural Khartoum, Omer et al. [Reference Omer, El Dawla, Nicolas, Roumiantzeff and Lapeyssonie22] found 41·7% of children had detectable MV-antibodies at 5 months, dropping sharply to nil at 8–9 months. In the same area as our cohort study, E. W. Hoskins [Reference Hoskins23] previously reported MV-specific plaque reduction neutralization titres of <50 mIU/ml in 19%, 50–100 mIU/ml in 34% and ⩾100 mIU/ml in 47% of 302 5-month-old infants sampled in 1989. Studies in other countries also showed that MV-specific neutralizing antibodies were present in 64, 39 and 2% of infants aged 6, 9, and 12 months respectively [Reference Gans, Yasukawa and Rinki6]. In Oman, Kohler et al. [Reference Kohler, Suleiman and Robertson24] found that only 8·9% of 881 9-month-old infants had detectable MV-antibodies. It is important to emphasize that in the present study all mothers had maternal immunity derived from natural MV infection. It has been shown previously that maternal antibody levels derived from vaccinated mothers are lower, which will increase the window of susceptibility [Reference Putz, Bouche, De Swart and Muller3].
Measles vaccination coverage provided by mobile EPI teams was reasonable: by age 2 years almost 80% of the infants had been vaccinated. However, similar to previous studies [Reference El Mubarak, Yuksel, Mustafa, Ibrahim, Osterhaus and De Swart9, Reference De Swart, El Mubarak and Vos25] the results of the serological analysis suggested low seroconversion levels in the infants. During the complete study period nine vaccinated infants were diagnosed with clinical measles. Although no samples were collected for laboratory confirmation, this indeed suggests a significant level of vaccination failure. In our study it was impossible to discriminate between vaccination-induced or infection-induced acquired antibodies, but the above- mentioned observations suggest that a substantial number of infants seroconverted or were boosted by natural infection. Further research is needed to evaluate vaccination coverage and efficacy in this area.
The present study demonstrates that blood samples collected on filter paper can effectively be used to monitor trends in specific antibody levels for seroepidemiological purposes. Although our study was prospective, the same approach could also be used in cross-sectional studies for evaluation of vaccination programmes in areas with limited infrastructure.
ACKNOWLEDGEMENTS
We thank the health visitor and nurse midwife, Fathia M. Ibrahim for training the midwives and supervising the blood sampling. We also thank the village midwives for their help and cooperation. We are grateful to the mothers and the children who participated in this study. Finally, we thank Professor A. M. El Hassan, Dr M. M. Mukhtar, Dr E. E. Zijlstra and Dr H. S. El Mubarak for their contributions to this study. This work was supported by INCO-DC grant IC18CT96-0116 from the European Commission.




