Article contents
Transmission Electron Microscopic Study of Coexisting Pyrophyllite and Muscovite: Direct Evidence for the Metastability of Illite
Published online by Cambridge University Press: 02 April 2024
Abstract
Transmission electron microscopy has been used to characterize coexisting pyrophyllite and muscovite in low-grade metamorphosed pelites from Witwatersrand and northeastern Pennsylvania. The Witwatersrand sample consisted largely of porphyroblasts of interlayered muscovite and pyrophyllite in a fine-grained matrix of the same phases. In both textures, muscovite and pyrophyllite occurred as interlayered packets (with apparently coherent interfaces) from about 300 Å to a few micrometers in thickness, with no mixed layering. Their compositions were determined with a scanning transmission electron microscope to be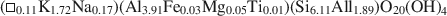
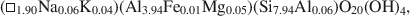
The pyrophyllite and muscovite in the Pennsylvania shale likewise occurred only as coexisting coherent to sub-parallel packets as thin as 200 Å, with compositions of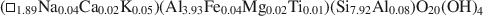
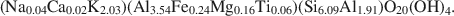
The compositions of these coexisting pyrophyllite and muscovite define a solvus with steep limbs and extremely limited solid solution. Illite is a white mica, intermediate in composition between pyrophyllite and muscovite, formed at much lower temperatures than muscovite. These relations show that illite is metastable relative to pyrophyllite + muscovite in all of its diagenetic and low-grade metamorphic occurrences. This further implies that illite precursor phases, such as smectite, are also metastable. The prograde reactions involving smectite, illite, and muscovite are therefore inferred to represent Ostwald-step-rule-like advances through a series of metastable phases toward the equilibrium states attained in the greenschist facies. “Illite crystallinity” can therefore be a measure of reaction progress, for which temperature is only one of several determining factors.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © 1990, The Clay Minerals Society
Footnotes
Contribution 467 from the Mineralogical Laboratory, Department of Geological Sciences, University of Michigan, Ann Arbor, Michigan 48109.
References
- 38
- Cited by




