Impact statement
Coastal habitats such as mangroves, tidal marshes and seagrasses have faced extensive loss due to human activities such as land use change and pollution. This loss occurs despite the value of these ecosystems as important reservoirs of ‘blue carbon’, with much of their carbon stored in the soil column. Recent interest has turned to blue carbon conservation and restoration, with managers and policy makers around the world setting targets to restore habitats to generate carbon credits or meet national targets for climate change mitigation that are larger in magnitude than targets previously set. This article collates the empirical evidence base for how restoration activities can positively impact various parts of the blue carbon cycle and contribute to climate change mitigation. However, carbon benefits will only happen if projects can overcome various socioeconomic, governance and biophysical constraints to restoration that currently limit our ability to restore coastal landscapes at the scale required to tackle the climate change challenge.
Introduction
Natural climate solutions – actions that protect, sustainably manage and restore ecosystems – are an important tool in mitigating climate change and keeping global temperatures below a 2 °C increase by the end of this century. Natural climate solutions, such as those provided by forests, wetlands, grasslands and agricultural lands, could provide one-third of the cost-effective climate mitigation needed to achieve this goal, equivalent to 23.8 Pg CO2 yr.−1 (Griscom et al., Reference Griscom, Adams, Ellis, Houghton, Lomax, Miteva, Schlesinger, Shoch, Siikamäki, Smith, Woodbury, Zganjar, Blackman, Campari, Conant, Delgado, Elias, Gopalakrishna, Hamsik, Herrero, Kiesecker, Landis, Laestadius, Leavitt, Minnemeyer, Polasky, Potapov, Putz, Sanderman, Silvius, Wollenberg and Fargione2017), while providing a wide range of co-benefits, such as food provision, livelihoods and cultural services to local communities.
A natural climate solution that has gained substantial interest over the last decade is the conservation and restoration of blue carbon. The current definition of blue carbon genereally refers to the carbon sequestered and stored in three specific coastal ecosystems: mangroves, tidal marshes and seagrasses. Mangroves are a community of salt-tolerant trees covering >145,000 km2 (Jia et al., Reference Jia, Wang, Mao, Ren, Song, Zhao, Wang, Xiao and Wang2023) in the tropics, subtropics and warm temperate regions. In some instances, they are able to store almost 2,800 Mg C ha−1 in soil layers 1–6 m deep (Adame et al., Reference Adame, Santini, Torres-Talamante and Rogers2021). Tidal marshes cover 53,000 km2 globally (Worthington et al., Reference Worthington, Spalding, Landis, Maxwell, Navarro, Smart and Murray2024), while estimates of global seagrass extent vary from ~160,000 km2 (McKenzie et al., Reference McKenzie, Nordlund, Jones, Cullen-Unsworth, Roelfsema and Unsworth2020) to ~1.65 million km2 (Jayathilake and Costello, Reference Jayathilake and Costello2018), depending on the methods used for mapping these systems. Together, these three ecosystems store >30,000 Tg C (Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021).
Despite their importance for climate change mitigation, blue carbon ecosystems have experienced substantial declines in area due to human land use change and coastline modification, and continue to be lost around the world (Fluet-Chouinard et al., Reference Fluet-Chouinard, Stocker, Zhang, Malhotra, Melton, Poulter, Kaplan, Goldewijk, Siebert and Minayeva2023). Mangroves were coarsely estimated to be lost at rates of 1–3% yr.−1 in the 20th century, although this has reduced to approximately 0.1–0.2% from 2000 onward (Friess et al., Reference Friess, Rogers, Lovelock, Krauss, Hamilton, Lee, Lucas, Primavera, Rajkaran and Shi2019). Tidal marshes have been lost at 0.28% yr.−1 in the 21st century (Campbell et al., Reference Campbell, Fatoyinbo, Goldberg and Lagomasino2022), and seagrasses are threatened across much of their global range, with losses of at least 5,602 km2 since 1880 (Dunic et al., Reference Dunic, Brown, Connolly, Turschwell and Côté2021). Habitat loss contributes to climate change through the emissions of stored carbon; in the 21st century, global mangrove loss led to the emissions of 26.3 Tg CO2e yr.−1 (Hamilton and Friess, Reference Hamilton and Friess2018), while tidal marsh loss released 16.3 Tg CO2e yr.−1 to the atmosphere (Campbell et al., Reference Campbell, Fatoyinbo, Goldberg and Lagomasino2022).
Lost areas of habitat provide an opportunity for new blue carbon accumulation through habitat restoration. In coastal wetlands, the term ‘restoration’ encompasses a range of management actions. They can generally be defined as the planting of seedlings (mangroves, Zimmer et al., Reference Zimmer, Ajonina, Amir, Cragg, Crooks and Dahdouh-Guebas2022; tidal marshes, Sparks et al., Reference Sparks, Cebrian, Biber, Sheehan and Tobias2013; seagrasses, van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016) or the encouragement of natural regeneration, often through the removal of environmental stressors or the reintroduction of hydrological flows (mangroves, Lewis, Reference Lewis2005; tidal marshes, Garbutt and Wolters, Reference Garbutt and Wolters2008; seagrasses, Bourderesque et al., Reference Bourderesque, Blanfuné, Pergent and Thibaut2021 ) and/or the broadcasting of seeds and propagules on high tides (e.g., Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020). Coastal restoration activities are attracting substantial recent interest and funding (UNEP-WCMC, 2022), and restoration is a management action that is now the basis of several blue carbon projects (Friess et al., Reference Friess, Howard, Huxham, Macreadie and Ross2022a).
In this review, we synthesize the experiences of the restoration of mangroves, tidal marshes and seagrasses for blue carbon outcomes. Specifically, we outline: (i) the global-scale biophysical potential for the restoration of blue carbon ecosystems; (ii) estimates of blue carbon benefits following coastal habitat restoration; and (iii) current challenges and constraints to the effective restoration of blue carbon ecosystems. We aim for this to be a broad synthesis of the potential benefits of restoration and challenges to their implementation, and we refer the reader to in-depth or systematic reviews on specific topics where appropriate.
Large-scale scope of restoration for blue carbon
Due to centuries of coastal habitat loss, a large extent globally of areas could be reverted to their original state, with concomitant gains in blue carbon. Macreadie et al. (Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021) estimate this area to be approximately 30 million ha (with a 95% confidence interval of 17.5–41.6 million ha), with seagrasses accounting for 57% and mangroves accounting for 37% of this potential. Tidal marshes have the lowest global restoration potential, probably because current land uses (such as urban development in estuaries) preclude conversion back to tidal marsh. If the restoration of blue carbon ecosystems could be conducted successfully at this scale, it could result in the removal of 841 Tg CO2e per year from the atmosphere, equivalent to ~3% of global fossil fuel emissions (Macreadie et al., Reference Macreadie, Costa, Atwood, Friess, Kelleway, Kennedy, Lovelock, Serrano and Duarte2021). Studies for mangroves specifically suggest that 8,120 km2 deforested since 1996 might be biophysically restorable (Worthington and Spalding, Reference Worthington and Spalding2018); resulting in 365 million tonnes of carbon gains once fully restored.
Such large potential carbon gains align with several international initiatives promoting large-scale coastal habitat restoration, such as the Trillion Trees Initiative and the Bonn Challenge (Lovelock et al., Reference Lovelock, Adame, Bradley, Dittmann, Hagger, Hickey, Hutley, Jones, Kelleway, Lavery and Macreadie2022), although interest and implementation from these initiatives in blue carbon ecosystems could still increase further to match that of terrestrial ecosystems (Waltham et al., Reference Waltham, Elliott, Lee, Lovelock, Duarte, Buelow, Simenstad, Nagelkerken, Claassens and Wen2020). Countries have also identified large-scale opportunities for blue carbon restoration. For example, Indonesia has a target of restoring 600,000 ha of mangroves by 2024, with carbon gains as a key driver of this policy (Sidik et al., Reference Sidik, Lawrence, Wagey, Zamzani and Lovelock2023). While studies suggest that only ~186,600 ha (Worthington and Spalding, Reference Worthington and Spalding2018) to 193,367 ha (Sasmito et al., Reference Sasmito, Basyuni, Kridalaksana, Saragi-Sasmito, Lovelock and Murdiyarso2023) are biophysically suitable for restoration in Indonesia, it shows the commitment that some countries have for blue carbon restoration. Other countries have set blue carbon restoration targets within their Nationally Determined Contributions to the Paris Agreement by 2030; in 2020–2021, Belize committed to restoring <4,000 ha of mangroves, Haiti committed to increasing its mangrove area to 19,500 ha and Samoa committed to increasing its mangrove area by 5% (Friess, Reference Friess2023).
Carbon benefits of restoration
The achievement of meaningful coastal restoration targets is expected to have important impacts on blue carbon cycling and storage (Figure 1). Restoration will have positive impacts on aboveground and belowground biomass pools, with carbon stocks increasing by 2–800 times, depending on the ecosystem and setting (Stagg and Mendelssohn, Reference Stagg and Mendelssohn2010; Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019; Oreska et al., Reference Oreska, McGlathery, Aoki, Berger, Berg and Mullins2020; Rosentreter et al., Reference Rosentreter, Al-Haj, Fulweiler and Williamson2021; Iram et al., Reference Iram, Maher, Lovelock, Baker, Cadier and Adame2022; Shao et al., Reference Shao, Han, Yang, Li, Zhang, Ma, Duan and Sun2022; Kelsall et al., Reference Kelsall, Quirk, Wilson and Snedden2023). Similar positive impacts are expected on the soil carbon pool (generally referring to the top 1 m of sediment), although patterns are more mixed due to legacy carbon from prior land uses. Positive impacts on carbon fluxes are generally expected. For example, methane emissions would be expected to be as much as four times lower than degraded sites or those under other land uses, particularly if the replaced habitat was dominated by freshwater, due to the influence of salinity on methanogenesis (e.g., Cotavicz et al., Reference Cotavicz, Abril, Sanders, Tait and Sippo2024).

Figure 1. Restored blue carbon stocks (aboveground and belowground, AGB and BGB) tend to be 2–800 times higher than in degraded/converted/bare sites, and methane (CH4) fluxes can be four times less than degraded/converted/bare sites, depending on habitats and age. For example, (a) seagrasses, bare versus (vs) restored (Oreska et al., Reference Oreska, McGlathery, Aoki, Berger, Berg and Mullins2020); (b) Mangrove, converted/degraded vs restored (Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019; Rosentreter et al., Reference Rosentreter, Al-Haj, Fulweiler and Williamson2021) and (c) tidal marshes; converted/degraded vs restored (Stagg and Mendelssohn, Reference Stagg and Mendelssohn2010; Iram et al., Reference Iram, Maher, Lovelock, Baker, Cadier and Adame2022; Shao et al., Reference Shao, Han, Yang, Li, Zhang, Ma, Duan and Sun2022; Kelsall et al., Reference Kelsall, Quirk, Wilson and Snedden2023); refer to the original references for details; positive values indicate an increase and negative values indicate a decrease.
The following describes major stock and flux components separately for each blue carbon ecosystem, and is indicative of the magnitude of stock and flux change that can be expected following restoration. For in-depth reviews of general carbon stocks and fluxes for different blue carbon ecosystems, we direct the reader to systematic reviews such as Fourqurean et al. (Reference Fourqurean, Duarte, Kennedy, Marbà, Holmer, Mateo, Apostolake, Kendrick, Krause-Jensen, McGlathery and Serrano2012) for seagrass stocks; Johannessen (Reference Johannessen2022) for seagrass carbon accumulation rates; Sasmito et al. (Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019) for carbon stock change and GHG flux change with mangrove restoration; Mason et al. (Reference Mason, Burden, Epstein, Jupe, Wood and Skov2023) for carbon accumulation rates and GHG fluxes for tidal marshes; and Taillardat et al. (Reference Taillardat, Thompson, Garneau and Friess2020) for GHG fluxes for multiple blue carbon ecosystems. For further information on geomorphic and ecological drivers of blue carbon stocks and fluxes, we direct the reader to a recent review by Kirwan et al. (Reference Kirwan, Megonigal, Noyce and Smith2023).
Mangroves
Mangrove restoration efforts vary in the degree of human intervention, and include ecological engineering, monoculture plantations, afforestation and ecological mangrove restoration that promotes natural regeneration (Ellison et al., Reference Ellison, Felson and Friess2020). The latter is encouraged through hydrological modification, including the construction or reconfiguration of tidal creeks, culverts, sediment additions to change elevation and tidal reintroduction (Lewis, Reference Lewis2005). Restoration projects are increasingly incorporating carbon benefits, and chronosequence studies are being used to understand how blue carbon dynamics change with restoration age (Osland et al., Reference Osland, Spivak, Nestlerode, Lessmann, Almario, Heitmuller, Russell, Krauss, Alvarez, Dantin, Harvey, From, Cormier and Stagg2012; Andreetta et al., Reference Andreetta, Huertas, Lotti and Cerise2016; Marchand, Reference Marchand2017; Walcker et al., Reference Walcker, Gandois, Proisy, Corenblit, Mougin, Laplanche, Ray and and Fromard2018; Wang et al., Reference Wang, Yu, Singh, Guan, Xiong, Zheng and Xiao2021; Azman et al., Reference Azman, Sharma, Liyana Hamzah, Mohamad Zakaria, Palaniveloo and MacKenzie2023) alongside systematic literature reviews (Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019; Rivera-Monroy et al., Reference Rivera-Monroy, Zhao, Wang and Xue2022) to estimate carbon outcomes from restoration.
Total ecosystem carbon stocks in restored mangroves generally increase with site age. For example, studies in southeast Australia indicated a strong relationship between carbon and site age (Carnell et al., Reference Carnell, Palacios, Waryszak, Trevathan-Tackett, Masqué and Macreadie2022), with stocks in older restored forests (17 and 35 years) averaging ~115 MgC ha−1, compared to only ~50 MgC ha−1 in younger forests (13 years) (Carnell et al., Reference Carnell, Palacios, Waryszak, Trevathan-Tackett, Masqué and Macreadie2022). In a restoration chronosequence study in Vietnam, total ecosystem carbon stocks also increased with stand age (2 to 27 years old) from ~201 to ~519 MgC ha−1 (Pham et al., Reference Pham, Luu, Nguyen and Koji2017). However, specific carbon pools may respond differently to restoration; meta-analyses suggest that mangrove biomass carbon increases for 15 years at a rate of 4 MgC ha−1 yr.−1 after restoration, although mixed patterns are observed in soil carbon stocks up to 1 m depth (Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019).
Although aboveground biomass can regenerate rapidly, carbon storage belowground takes substantially longer to replenish, with shallow soil carbon pools restocked more quickly than deeper layers due to organic matter and allochthonous inputs. A study in Thailand of hydraulic restoration and planting following conversion to shrimp ponds reported that soil organic carbon concentration increased over 4 years and stocks increased from 110 to 160 MgC ha−1 in 2 years, based on measurements at a 5 cm soil depth (Matsui et al., Reference Matsui, Suekuni, Nogami, Havanond and Salikul2010). In created mangroves along a 20-year chronosequence in southwest Florida, soil organic matter and total carbon increased with site age in the upper 10 cm of the soil and were estimated to need 18–28 years to reach natural equivalence (Osland et al., Reference Osland, Spivak, Nestlerode, Lessmann, Almario, Heitmuller, Russell, Krauss, Alvarez, Dantin, Harvey, From, Cormier and Stagg2012). Total carbon in the upper 10 cm soil layer was 30 g kg−1 in the created wetland compared to 144 g kg−1 in the natural wetland, and 13 g kg−1 and 88 g kg−1 in the lower 10–30 cm layer in the created and natural wetlands (Osland et al., Reference Osland, Spivak, Nestlerode, Lessmann, Almario, Heitmuller, Russell, Krauss, Alvarez, Dantin, Harvey, From, Cormier and Stagg2012). Other studies have observed that soil organic carbon increases with restoration activities (Zhang et al., Reference Zhang, Shen, Ren, Wang and Han2012; Dung et al., Reference Dung, Tue, Nhuan and Omori2016; Pham et al., Reference Pham, Luu, Nguyen and Koji2017; Sasmito et al., Reference Sasmito, Taillardat, Clendenning, Cameron, Friess, Murdiyarso and Hutley2019; Ratul et al., Reference Ratul, Gu, Qiao, Sagala, Nan, Islam and Chen2022; Thura et al., Reference Thura, Serrano, Gu, Fang, Htwe, Zhu, Huang, Agusti, Duarte, Wang and Wu2023). Restoration activity can also influence soil carbon returns; a global review found that restoration was more effective at accumulating carbon in the top meter of soil over 40 years compared to afforestation projects (Song et al., Reference Song, Ding, Li, Meng, Zhou, Gou, Zhang, Ye, Saintilan, Krauss, Crooks, Lv and Lin2023). In some cases, carbon stocks in restored mangroves can exceed those in natural systems, but differences in these comparisons may be attributed to local hydrogeomorphological controls on carbon storage (Kusumaningtyas et al., Reference Kusumaningtyas, Kepel, Solihuddin, Lubis, Putra, Sugiharto, Ati, Salim, Mustikasari, Heriati, Daulat, Sudirman, Suryono and Rustam2022).
While most research has focused on changes in carbon stocks over time, we lack a clear understanding of how carbon sequestration and greenhouse gas fluxes respond to mangrove restoration. Limited evidence from southeast Australia showed that sequestration rates do respond to site age, at ~3 Mg C ha−1 yr.−1 in older forests compared to ~1.5 Mg C ha−1 yr.−1 in younger forests (Carnell et al., Reference Carnell, Palacios, Waryszak, Trevathan-Tackett, Masqué and Macreadie2022). Methane fluxes decreased with age, being ~5 times higher in the younger forest compared to older forests, with rates of 5.8 mg CH4 m−2 day−1 (Carnell et al., Reference Carnell, Palacios, Waryszak, Trevathan-Tackett, Masqué and Macreadie2022). Flux measurements are important to understand the climate change mitigation potential of mangrove restoration; in an analysis of mangrove restoration offset potential in North Sulawesi, Indonesia, carbon mitigation was estimated at −27.6 Mg CO2-e ha−1 yr.−1 (Cameron et al., Reference Cameron, Hutley, Friess and Brown2019). Scaled up to the national level in Indonesia, this could represent an offset potential of up to 16.56 million Mg CO2-e ha−1 yr.−1 (Cameron et al., Reference Cameron, Hutley, Friess and Brown2019).
Tidal marshes
There is global interest in restoring tidal marshes to recover lost ecosystem functions and services (Macreadie et al., Reference Macreadie, Nielsen, Kelleway, Atwood, Seymour, Petrou, Connolly, Thomson, Trevathan-Tackett and Ralph2017; Kelleway et al., Reference Kelleway, Serrano, Baldock, Burgess, Cannard, Lavery, Lovelock, Macreadie, Masqué, Newnham and Saintilan2020; Hagger et al., Reference Hagger, Waltham and Lovelock2022). Tidal marshes are often restored in areas with high salinity (>15 ppt), and can be colonized by a range of plant species adapted to those conditions (Craft, Reference Craft2007; Negandhi et al., Reference Negandhi, Edwards, Kelleway, Howard, Safari and Saintilan2019). Several techniques are used to restore tidal marshes (Billah et al., Reference Billah, Bhuiyan, Islam, Das and Hoque2022; Craft, Reference Craft2022; Mason et al., Reference Mason, Burden, Epstein, Jupe, Wood and Skov2023) that can enhance carbon sequestration (Macreadie et al., Reference Macreadie, Nielsen, Kelleway, Atwood, Seymour, Petrou, Connolly, Thomson, Trevathan-Tackett and Ralph2017; Kelleway et al., Reference Kelleway, Serrano, Baldock, Burgess, Cannard, Lavery, Lovelock, Macreadie, Masqué, Newnham and Saintilan2020). Common techniques include the placement of dredge material on subsiding marshes or construction of new marshes by filling open water areas (Costa-Pierce and Weinstein, Reference Costa-Pierce and Weinstein2002; Madrid et al., Reference Madrid, Quigg and Armitage2012). Hydrology, including salinity, tidal flow and water level conditions, are major drivers influencing tidal marsh productivity and the success of restoration. Hydrological restoration involves diverting river water (Baustian et al., Reference Baustian, Liu, Moss, Dausman and Pahl2023) and de-embankment of existing dikes or enlarging culverts (Wolters et al., Reference Wolters, Garbutt and Bakker2005; Karberg et al., Reference Karberg, Beattie, O’Dell and Omand2018). Tidal reinstatement is used for restoring marshes on marginal agricultural lands in coastal areas (Kelleway et al., Reference Kelleway, Serrano, Baldock, Burgess, Cannard, Lavery, Lovelock, Macreadie, Masqué, Newnham and Saintilan2020; Lovelock et al., Reference Lovelock, Adame, Bradley, Dittmann, Hagger, Hickey, Hutley, Jones, Kelleway, Lavery and Macreadie2022), reintroducing tidal connections through the installation, removal or modification of water regulation structures.
The time needed for tidal marsh restoration to achieve ecological equivalence to natural marshes can vary between 3 and >15 years (Broome et al., Reference Broome, Craft and Burchell2019; Billah et al., Reference Billah, Bhuiyan, Islam, Das and Hoque2022), but significant increases in carbon stocks (up to 3 times; Shao et al., Reference Shao, Han, Yang, Li, Zhang, Ma, Duan and Sun2022) can be reached as early as 4 years (O’Connor et al., Reference O’Connor, Fest, Sievers and Swearer2020). Annual aboveground carbon production was 200–1,700 g C m−2 in constructed wetlands of the United States (from placement of dredged material) after 2–3 years (Madrid et al., Reference Madrid, Quigg and Armitage2012). Restored wetlands in China also had aboveground biomass stocks near 167 g C m−2, which were three times higher than degraded wetlands (Shao et al., Reference Shao, Han, Yang, Li, Zhang, Ma, Duan and Sun2022). The total carbon soil stock (to 1 m) of restored marshes can be about 51.86 Mg C ha−1, approximately two times higher than that of degraded wetlands (Shao et al., Reference Shao, Han, Yang, Li, Zhang, Ma, Duan and Sun2022).
Annual fluxes are often measured and combined to assess net carbon (or CO2 equivalence) benefits (Baustian et al., Reference Baustian, Liu, Moss, Dausman and Pahl2023). Aboveground and belowground net primary production rates of restored marshes in the United States can increase by four times (depending on sediment treatment) compared to degraded sites (Stagg and Mendelssohn, Reference Stagg and Mendelssohn2010). Soil carbon accumulation rates vary in restored tidal marshes in comparison to natural marshes, with some studies indicating the rates are lower than those of natural marshes (Broome et al., Reference Broome, Craft and Burchell2019, Kelsall et al., Reference Kelsall, Quirk, Wilson and Snedden2023, Table 1), whereas other studies indicated soil carbon accumulation rates of restored salt marshes can be nearly twice as high as reference marshes (Poppe and Rybczyk, Reference Poppe and Rybczyk2021; Mason et al., Reference Mason, Burden, Epstein, Jupe, Wood and Skov2023). The direction of this relationship may be somewhat independent of restoration, and more influenced by biophysical processes and plant species composition (Mason et al., Reference Mason, Burden, Epstein, Jupe, Wood and Skov2023).
Table 1. Indicative carbon abatement benefits (g C m−2 yr−1) from soil carbon accumulation rates (from 10 to 30 cm depths) and greenhouse gas fluxes of tidal marsh restoration (see references for details on marsh ages, geomorphic settings etc.)

Note: For a global review of carbon fluxes of restored salt marshes, see also Mason et al. (Reference Mason, Burden, Epstein, Jupe, Wood and Skov2023).
Anoxic soil conditions promote greenhouse gas emissions, reducing the magnitude of carbon abatement benefits (Emery and Fulweiler, Reference Emery and Fulweiler2017). Emissions of CO2 or CH4 from restored tidal marshes are highly variable and influenced by the legacy of restored soil (Iram et al., Reference Iram, Maher, Lovelock, Baker, Cadier and Adame2022), hydrology, vegetation (Derby et al., Reference Derby, Needelman, Roden and Megonigal2022) and elevation levels (Negandhi et al., Reference Negandhi, Edwards, Kelleway, Howard, Safari and Saintilan2019). Emissions are also strongly influenced by salinity regime (Poffenbarger et al., Reference Poffenbarger, Needelman and Megonigal2011; Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017). Restored tidal marshes are considered most effective in providing carbon abatement because microbial communities are influenced by salinity and tidal exchange, resulting in reduced greenhouse gas fluxes (Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017; Negandhi et al., Reference Negandhi, Edwards, Kelleway, Howard, Safari and Saintilan2019). Restored tidal marshes had CO2 and CH4 fluxes (Adams et al., Reference Adams, Andrews and Jickells2012; Burden et al., Reference Burden, Garbutt, Evans, Jones and Cooper2013; Negandhi et al., Reference Negandhi, Edwards, Kelleway, Howard, Safari and Saintilan2019; Iram et al., Reference Iram, Maher, Lovelock, Baker, Cadier and Adame2022; Table 1) within the range of reference marshes and global averages for natural saltmarshes (Poffenbarger et al., Reference Poffenbarger, Needelman and Megonigal2011; Kroeger et al., Reference Kroeger, Crooks, Moseman-Valtierra and Tang2017; Rosentreter et al., Reference Rosentreter, Al-Haj, Fulweiler and Williamson2021). However, high precipitation events cause freshening, thus buffering salinity and reducing the carbon abatement potential of restored salt marshes during such events (Negandhi et al., Reference Negandhi, Edwards, Kelleway, Howard, Safari and Saintilan2019).
Seagrasses
Seagrass restoration has been conducted for many decades (van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016; Ward and Beheshti, Reference Ward and Beheshti2023). Although the scale of restoration is often small compared to other coastal ecosystems, newer techniques in seed-based restoration (Tan et al., Reference Tan, Dalby, Kendrick, Statton, Sinclair, Fraser, Macreadie, Gillies, Coleman and Waycott2020) and mimicry of emergent traits (Temmink et al., Reference Temmink, Christianen, Fivash, Angelini, Boström, Didderen, Engel, Esteban, Gaeckle and Gagnon2020) can facilitate large-scale planting and increase restoration effectiveness (van Katwijk et al., Reference van Katwijk, Bos, de Jonge, Hanssen, Hermus and de Jong2009). Several guidelines focus on seagrass restoration at regional (e.g., Western Indian Ocean Region) or national (e.g., Sweden, Kiribati, United Kingdom and Ireland) scales (e.g., UNEP, 2020; Gamble et al., Reference Gamble, Debney, Glover, Bertelli and Green2021).
Although successful seagrass restorations have been documented (van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016; Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020; Tan et al., Reference Tan, Dalby, Kendrick, Statton, Sinclair, Fraser, Macreadie, Gillies, Coleman and Waycott2020), few report carbon stock recovery and sequestration in restored meadows. Those that have been assessed for carbon recovery primarily occur in the temperate coastal bays of Virginia in the mid-Atlantic, United States, the subtropical/tropical Gulf of Mexico, United States and temperate southern Australia (Table 2). Measurements here show that: (i) restored meadows have comparable or higher carbon burial rates and surface sediment carbon stocks compared to mature meadows (McGlathery et al., Reference McGlathery, Reynolds, Cole, Orth, Marion and Schwarzschild2012; Marbà et al., Reference Marbà, Arias-Ortiz, Masqué, Kendrick, Mazarrasa, Bastyan, Garcia-Orellana and Duarte2015; Thorhaug et al., Reference Thorhaug, Poulos, López-Portillo, Ku and Berlyn2017; Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020); (ii) estimates can be made regarding timing, trajectory, spatial patterns and sources of blue carbon recovery following seagrass restoration (McGlathery et al., Reference McGlathery, Reynolds, Cole, Orth, Marion and Schwarzschild2012; Greiner et al., Reference Greiner, McGlathery, Gunnell and McKee2013, Reference Greiner, Wilkinson, McGlathery and Emery2016; Oreska et al., Reference Oreska, McGlathery and Porter2017a,Reference Oreska, Wilkinson, McGlathery, Bost and McKeeb) and (iii) there are impacts of disturbance and natural recovery on carbon (Marbà et al., Reference Marbà, Arias-Ortiz, Masqué, Kendrick, Mazarrasa, Bastyan, Garcia-Orellana and Duarte2015; Thorhaug et al., Reference Thorhaug, Poulos, López-Portillo, Ku and Berlyn2017).
Table 2. Summary of specific studies investigating seagrass carbon benefits through habitat restoration

In Virginia, where seagrass meadows have been restored via seed broadcasting since 2001 (McGlathery et al., Reference McGlathery, Reynolds, Cole, Orth, Marion and Schwarzschild2012; Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020), carbon stocks in the top 5 cm of sediment in the meadow were twice that of adjacent bare sediments after 9 years (278.9 compared to 138.7 g C m−2; McGlathery et al., Reference McGlathery, Reynolds, Cole, Orth, Marion and Schwarzschild2012) and 1.3× greater than that of younger (1–5 year old) areas of the meadow (Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020). The carbon burial rate was significantly higher in 10-year-old meadows (36.68 g C m−2 y−1) compared to 4-year-old meadows and unvegetated areas (no net accumulation), with a 5-year lag period after seeding before carbon burial rates doubled. Sequestration rates were expected to reach functional equivalence to mature meadows by year 12 after seeding based on projections of continued increases in shoot density (Greiner et al., Reference Greiner, McGlathery, Gunnell and McKee2013). This observed and expected increase in carbon sequestration occurred in parallel to a fining of the sediments and increases in shoot density with age. However, 12 years after restoration, the distribution of Corg concentrations at the meadow scale was driven by hydrodynamics, rather than the age of the seagrass within the meadow, with higher Corg concentration further into the meadow away from the bare subtidal edge due to current attenuation (Oreska et al., Reference Oreska, McGlathery and Porter2017a). Stable isotope analysis indicated that the sources of sediment organic matter in the restored seagrass meadows were distinct from those of bare sediment, and were on average composed of ~40–50% from seagrass and 46–56% from benthic macroalgae and/or seston produced in situ, with only ~3–10% coming from macroalgae or adjacent Spartina alterniflora salt marshes in restored meadows spanning 4–13 years old (Greiner et al., Reference Greiner, Wilkinson, McGlathery and Emery2016; Oreska et al., Reference Oreska, Wilkinson, McGlathery, Bost and McKee2017b).
In southwest Australia, carbon burial rates in restored meadows also increased with age, and reached functional equivalence to mature meadows 18 years after planting (mean carbon burial rate 26.4 ± 0.8 g C m−2 y−1; Marbà et al., Reference Marbà, Arias-Ortiz, Masqué, Kendrick, Mazarrasa, Bastyan, Garcia-Orellana and Duarte2015). In the Gulf of Mexico, seagrass meadows restored in eight previously disturbed areas of seagrass loss had higher 20-cm-deep sediment carbon stocks (mean Corg stocks 38.7 ± 13.1 Mg ha−1) than impacted barren or always barren sediments, with the highest carbon stocks in older restored beds. These values were comparable to or higher than natural seagrass meadows, suggesting that seagrass restoration can reduce carbon losses from previous disturbances to seagrass meadows (Thorhaug et al., Reference Thorhaug, Poulos, López-Portillo, Ku and Berlyn2017). Mean organic carbon gains in the top 20 cm of sediment in restored sites, compared to impacted (now barren) sites, were estimated as 20.96 Mg Corg ha−1; however, carbon accumulation rates varied significantly by site and restoration age (but not species), with intermediate-aged seagrasses (4–15 years old) having the highest carbon accumulation rates compared to young (<3 years old) or old (>15 years old) restored seagrasses (Thorhaug et al., Reference Thorhaug, Poulos, López-Portillo, Ku and Berlyn2017).
A complete understanding of the greenhouse gas offset potential of seagrass restoration requires a full inventory of carbon fluxes. The first complete inventory for a restored meadow (in Virginia) estimated net offsets of 0.42 t CO2e ha−1 yr.−1, for which the financial benefit based on carbon sequestration would only cover about 10% of restoration costs, suggesting additional ecosystem services should be assessed for incentivization (Oreska et al., Reference Oreska, McGlathery, Aoki, Berger, Berg and Mullins2020). Recent multidisciplinary modeling approaches have combined extensive knowledge of species’ growth and the Corg benefits through restoration (Duarte et al., Reference Duarte, Sintes and Marbà2013; Reynolds et al., Reference Reynolds, Waycott, McGlathery and Orth2016), which can demonstrate the long-term potential of seagrass restoration to capture and store carbon.
One challenge in understanding carbon dynamics following seagrass restoration is geographical biases in data collection and availability, with restoration projects mainly documented in the temperate and subtropical coastlines of North America, Europe, East Asia and Australia (van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016), and blue carbon benefits only recorded in studies in the United States and Australia (Table 1). This is despite key academic and management interest in restoring tropical seagrasses for climate change mitigation (Rifai et al., Reference Rifai, Quevedo, Lukman, CFA, Risandi, Hernawan, Uchiyama, Ambo-Rappe and Kohsaka2023). Similarly, most studies focused on the restoration of temperate species, with a single study assessing Corg benefits of restoration of tropical species (Table 2). In tropical regions, several seagrass species co-occur within one meadow, adding further complexity to restoration. The traits of tropical species are highly variable, from fast-growing colonizing species to long-lived persistent species, with different responses to disturbance (Kilminster et al., Reference Kilminster, McMahon, Waycott, Kendrick, Scanes, McKenzie, O’Brien, Lyons, Ferguson, Maxwell, Glasby and Udy2015). Multispecies restoration, such as a combination of the five species that include all species traits (colonizing, opportunistic and persistent), has been shown to have the highest restoration potential in Indonesia (Williams et al., Reference Williams, Ambo-Rappe, Sur, Abbott and Limbong2017; Asriani et al., Reference Asriani, Ambo-Rappe, Lanuru and Williams2018), and the combined restoration of colonizing and persistent species resulted in high organic carbon stocks in the Gulf of Mexico across 15–16 years and 38–43 years since restoration (Thorhaug et al., Reference Thorhaug, Poulos, López-Portillo, Ku and Berlyn2017).
While seagrass restoration can restore carbon sequestration processes (Table 2), many studies have not demonstrated additionality – the additional carbon generated as a result of an intended management action, which is a prerequisite for the calculation of carbon credits. Most restoration projects measure success based on metrics related to habitat attributes (e.g., shoot density) rather than the return of ecosystem services (Orth et al., Reference Orth, Lefcheck, McGlathery, Aoki, Luckenbach, Moore, Oreska, Snyder, Wilcox and Lusk2020). For studies that do measure changes in carbon, some approaches (such as radionuclides to determine soil carbon accumulation rates) require years of sediment accumulation, and natural seagrass sediment dynamics such as resuspension and mixing can prevent the estimation of sediment and carbon accumulation rates (Lafratta et al., Reference Lafratta, Serrano, Masqué, Mateo, Fernandes, Gaylard and Lavery2020). Other methods of measuring sediment accumulation to quantify carbon sequestration in seagrass meadows have proven difficult, such as modifications to the Rod Surface Elevation Table method to apply it to seagrass meadows, due to problems with measuring surface elevation change below the water surface, and maintaining feldspar marker horizons (Potouroglou et al., Reference Potouroglou, Bull, Krauss, Kennedy, Fusi, Daffonchio, Mangora, Githaiga, Diele and Huxham2017; but see Ewers Lewis and McGlathery, Reference Ewers Lewis and McGlathery2023). Quantification of changes in carbon dynamics is important to measure because not all restored meadows accumulate substantial volumes of additional carbon. Meadows exposed to high hydrodynamic energy may lack sediment accumulation, which can cause sediment mixing and erosion to be present decades after restoration/recovery (Lafratta et al., Reference Lafratta, Serrano, Masqué, Mateo, Fernandes, Gaylard and Lavery2020). Finally, uncertainties surrounding the influence of restoration on emissions of CH4, N2O and CO2, represent a global knowledge gap (Oreska et al., Reference Oreska, McGlathery, Aoki, Berger, Berg and Mullins2020), and accounting for emissions may result in low carbon benefits for some seagrass restoration projects.
Challenges to successful restoration
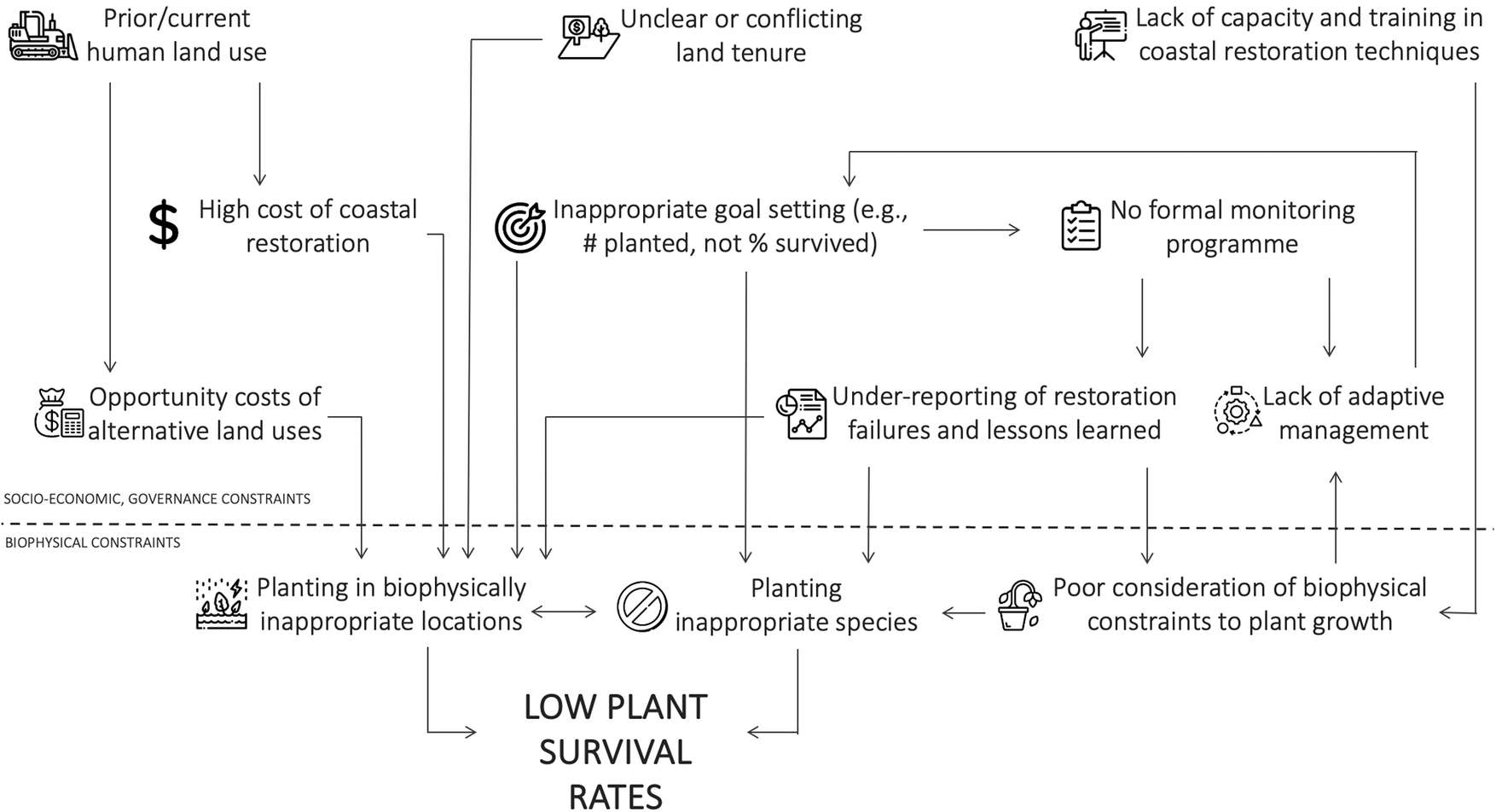
Regardless of restoration technique, blue carbon benefits will only be realized if restoration is conducted successfully. All restoration techniques ultimately require correct biophysical conditions within the site to allow successful planted or natural seedling establishment. While data availability on restoration outcomes is generally poor (Eger et al., Reference Eger, Earp, Friedman, Gatt, Hagger, Hancock, Kaewsrikhaw, Mcleod, Moore and Niner2022; Gatt et al., Reference Gatt, Andradi-Brown, Ahmadia, Martin, Sutherland, Spalding, Donnison and Worthington2022), the global track record in restoring blue carbon ecosystems is considered to be mixed. For example, most mangrove projects in Sri Lanka showed 0% survival, with only three sites >50% (Kodikara et al., Reference Kodikara, Mukherjee, Jayatissa, Dahdouh‐Guebas and Koedam2017). Similarly, in Colombia, only 24% of the projects were highly successful (Rodríguez-Rodríguez et al., Reference Rodríguez-Rodríguez, Mancera-Pineda and Tavera2021). For seagrass projects along the United States west coast, ~60% were unsuccessful (Ward and Beheshti, Reference Ward and Beheshti2023). Restoration, whether through planting or the encouragement of natural regeneration, is challenging because seedling mortality is generally high even in natural intertidal systems (e.g., van Regteren et al., Reference van Regteren, Meesters, Baptist, De Groot, Bouma and Elschot2020; Sloey et al., Reference Sloey, Lim, Moore, Heng, Heng and Van Breugel2022), and seedlings are often not planted in biophysically suitable conditions. Restoration attempts in suboptimal locations occur primarily due to socioeconomic and governance reasons (Friess et al., Reference Friess, Gatt, Ahmad, Brown, Sidik and Wodehouse2022b; Figure 2). This could include conflicting land tenure claims in suitable areas, pushing restoration activities into lower intertidal areas with fewer tenure conflicts, or ambitious short-term planting targets that require large extents of plantable areas to be found at short notice (e.g., Wodehouse and Rayment, Reference Wodehouse and Rayment2019).

Figure 2. Interlinked socioeconomic, governance and biophysical constraints can lead to low blue carbon restoration success.
Biophysical constraints to blue carbon restoration
The mortality of seedlings will be high if biophysical conditions are unsuitable for establishment. Generally, this means the physical environment of a restoration site must match the original ecohydrological conditions before disturbance. In mangroves and tidal marshes, this often relates to changes in the hydroperiod that are currently unsuitable wetland plants. For example, site elevations change substantially when aquaculture ponds are dug out, or when reclaimed areas are disconnected from further sediment input compared to the surrounding intertidal area. Other site conditions constraining wetland vegetation colonization include high sediment consolidation and low microtopography (Brooks et al., Reference Brooks, Mossman, Chitty and Grant2015).
Once the physical factors constraining natural reestablishment are understood, a key management step in wetland restoration is the removal or alleviation of that biophysical stressor (Lewis, Reference Lewis2005). In intertidal projects, these are often site-based engineering steps, such as manipulating site elevations through sediment addition (e.g., Staver et al., Reference Staver, Stevenson, Cornwell, Nidzieko, Staver, Owens, Logan, Kim and Malkin2020), and strategic dyke breaching (e.g., Kiesel et al., Reference Kiesel, Schuerch, Christie, Möller, Spencer and Vafeidis2020; López‐Portillo et al., Reference López‐Portillo, Zaldívar‐Jiménez, Lara‐Domínguez, Pérez‐Ceballos, Bravo‐Mendoza, Álvarez, Aguirre‐Franco, Krauss, Zhu and Stagg2021) to allow adequate water flows. However, removing constraints to natural reestablishment can be particularly challenging in seagrass restoration, as this ecosystem is often affected by stressors that occur at a distance from the restoration site, such as eutrophication caused by organic pollution from the surrounding watershed (van Katwijk et al., Reference van Katwijk, Thorhaug, Marbà, Orth, Duarte, Kendrick, Althuizen, Balestri, Bernard, Cambridge, Cunha, Durance, Giesen, Han, Hosokawa, Kiswara, Komatsu, Lardicci, Lee, Meinesz, Nakaoka, O’Brien, Paling, Pickerell, Ransijn and Verduin2016). Lack of freshwater input due to impoundment and manipulation of the wider watershed has also been a constraint to successful coastal wetland vegetation establishment (Liu et al., Reference Liu, Fagherazzi, Li and Cui2021).
Socioeconomic and governance constraints to blue carbon restoration
While biophysical variables determine the successful establishment of individual plants, the reasons why restoration projects are placed in suboptimal conditions in the first place are often the consequence of broader socioeconomic and governance constraints. Specifically, current land use may prohibit restoration back to the original blue carbon ecosystem; coastlines reclaimed for urban development have substantially less restoration potential than other land uses, for example (Worthington et al., Reference Worthington, zu Ermgassen, Friess, Krauss, Lovelock, Thorley, Tingey, Woodroffe, Bunting, Cormier, Lagomasino, Lucas, Murray, Sutherland and Spalding2020).
For intertidal areas where human use has been abandoned, restoration (particularly in tropical regions) can be inhibited by unclear or conflicting land tenure. Clarifying the land tenure landscape along many coastlines involves substantial investigation and negotiation, which will take time that may not be available for a project, pushing restoration efforts to locations with fewer land tenure concerns, such as commons or state lands in the subtidal zone that are not suitable for plant growth (Lovelock and Brown, Reference Lovelock and Brown2019; Friess et al., Reference Friess, Gatt, Ahmad, Brown, Sidik and Wodehouse2022b). Land tenure can be an obstacle to restoration even in developed bureaucracies. For example, challenges are faced when negotiating permission from private landholders, persuading risk-averse public stakeholders that restoration on their lands will be successful, or where ownership boundaries on the coast are still ambiguous (Bell-James et al., Reference Bell-James, Fitzsimons and Lovelock2023).
Inappropriate goal setting can also lead to poor restoration outcomes (Waltham et al., Reference Waltham, Alcott, Barbeau, Cebrian, Connolly, Deegan, Dodds, Goodridge Gaines, Gilby, Henderson, McLuckie, Minello, Norris, Ollerhead, Pahl, Reinhardt, Rezek, Simenstad, Smith, Sparks, Staver, Ziegler and Weinstein2021), such as planting in inappropriate locations. Many projects set the number of seedlings planted as a goal. Planting over the short time scales mandated by funders may encourage managers to seek large open areas (such as undervalued tidal flats, sensu Chen and Lee, Reference Chen and Lee2022) that provide sufficient space for planting but are not biophysically suitable for vegetation establishment (Lovelock and Brown, Reference Lovelock and Brown2019; Wodehouse and Rayment, Reference Wodehouse and Rayment2019). Ultimately, coastal restoration will be most successful when targets are based on robust knowledge of past and future changes that underpin suitable scenarios of environmental performance (Sheaves et al., Reference Sheaves, Waltham, Benham, Bradley, Mattone, Diedrich, Sheaves, Sheaves, Hernandez and Dale2021), which allows for long-term, iterative and adaptive management (e.g., Thom, Reference Thom2000).
Finally, coastal restoration costs can be high compared to terrestrial restoration, due to costs such as construction actions for hydrological manipulation (Bayraktarov et al., Reference Bayraktarov, Saunders, Abdullah, Mills, Beher, Possingham, Mumby and Lovelock2016). Carbon accounting of a rehabilitated seagrass meadow in the South Bay, Virginia, United States suggests that blue carbon credit generation may only offset ~10% of restoration costs (Oreska et al., Reference Oreska, McGlathery, Aoki, Berger, Berg and Mullins2020). Costs can sometimes be reduced by community labor for planting and other activities (e.g., Tan et al., Reference Tan, Dalby, Kendrick, Statton, Sinclair, Fraser, Macreadie, Gillies, Coleman and Waycott2020), although this is not often the major cost in a restoration project. As such, blue carbon projects that involve restoration are currently not breaking even, and future projects will likely require cofinancing options such as tax incentives or philanthropic funding in addition to carbon credit sales (Friess et al., Reference Friess, Howard, Huxham, Macreadie and Ross2022a). Combined, socioeconomic and governance factors have important implications for the scale of restoration; the area biophysically suitable for mangrove restoration in Southeast Asia is estimated at >303,000 ha, but only 5.5–34.2% of this area was deemed restorable once financial, land use and operational constraints were considered (Zeng et al., Reference Zeng, Sarira, Carrasco, Chong, Friess, Lee, Taillardat, Worthington, Zhang and Koh2020).
Spatial prioritization of blue carbon restoration
Many restoration projects are opportunistic in their location (e.g., Ledoux et al., Reference Ledoux, Cornell, O’Riordan, Harvey and Banyard2005) due to low land availability and opportunity costs. However, achieving ambitious restoration targets and meaningful blue carbon gains requires landscape-scale planning. Modeling can consider various biophysical, socioeconomic and governance constraints when determining optimal restoration locations (e.g., Syahid et al., Reference Syahid, Sakti, Ward, Rosleine, Windupranata and Wikantika2023), and has been widely used in predicting potential changes in the distribution of mangroves (Hu et al., Reference Hu, Wang, Zhang, Yu, Chen, Xie, Liu, Ma, Du, Chao, Lei and Chen2020; Rodriguez-Medina et al., Reference Rodriguez-Medina, Yanez-Arenas, Peterson, Euan Avila and Herrera-Silveira2020; Sahana et al., Reference Sahana, Areendran and Sajjad2022), seagrasses (Bertelli et al., Reference Bertelli, Stokes, Bull and Unsworth2022) and tidal marshes (Boumans et al., Reference Boumans, Burdick and Dionne2002; Raw et al., Reference Raw, Adams, Bornman, Riddin and Vanderklift2021). Species distribution models have been specifically used to determine suitable areas for seagrass (Valle et al., Reference Valle, Borja, Chust, Galparsoro and Garmendia2011; Stankovic et al., Reference Stankovic, Kaewsrikhaw, Rattanachot and Prathep2019) and mangrove (Hu et al., Reference Hu, Wang, Zhang, Yu, Chen, Xie, Liu, Ma, Du, Chao, Lei and Chen2020) restoration. These models predict the environmental distribution of species and can be projected into geographical space as habitat suitability, probability of species occurrence or favorability of species occurrence, which can be interpreted as restoration potential (Stankovic et al., Reference Stankovic, Kaewsrikhaw, Rattanachot and Prathep2019). The positive correlation of the models’ habitat suitability prediction and the planting units of seagrass (Valle et al., Reference Valle, Garmendi, Chust, Franco and Borja2015) demonstrated that usage of the model can eliminate “best professional judgment” for site selection (Stankovic et al., Reference Stankovic, Kaewsrikhaw, Rattanachot and Prathep2019). However, most models rely on complex relationships of the abiotic factors that shape a realized niche at a specific point in time, rather than the fundamental niche (Grech and Coles, Reference Grech and Coles2010), which can provide greater potential extents and additional restoration sites, resulting in an increase in total seagrass coverage (Oreska et al., Reference Oreska, McGlathery, Wiberg, Orth and Wilcox2021). In addition, these models, coupled with spatial drivers and models of blue carbon variability (e.g., Ewers Lewis et al., Reference Ewers Lewis, Young, Ierodiaconou, Baldock, Hawke, Sanderman, Carnell and Macreadie2019), offer guidelines for the return of the ecosystem services through restoration across spatial and temporal scales.
Modeling can also be used to show how the realized/fundamental niche may be modified as physical conditions change with climate change, such as increasing inundation periods under sea-level rise. This approach has most commonly been applied to conservation projects by assessing the sustainability of existing blue carbon resources and their projected distributions in the future (e.g., Rosencranz et al., Reference Rosencranz, Thorne, Buffington, Overton and Takekawa2019 for tidal marshes; Nguyen et al., Reference Nguyen, Friess, Todd, Mazor and Lovelock2022 for seagrasses and mangroves). Restoration suitability is implicit in these models when they predict landward migration into presently terrestrial areas, as those areas will need to be colonized by new intertidal vegetation. While less common, intertidal habitat restoration has also been specifically modeled under sea-level rise, with biogeomorphic models of a planned tidal marsh restoration project showing that newly colonizing vegetation will be able to keep pace with realistic rates of projected sea-level rise, and resilience is particularly influenced by restoration method (Gourgue et al., Reference Gourgue, van Belzen, Schwarz, Vandenbruwaene and Vanlede2022).
Conclusions
Extensive habitat loss creates ample opportunity for the restoration of mangroves, tidal marshes and seagrasses. The evidence is clear that their restoration leads to substantial per hectare gains in blue carbon, and has led to rapid interest in blue carbon restoration for the achievement of climate change mitigation policy. While the realizable area of restoration globally is much smaller than its biophysical potential, future blue carbon restoration is likely to make a meaningful contribution to climate change mitigation, while providing a wide range of co-benefits to coastal communities around the world.
The important carbon gains and other co-benefits from blue carbon restoration will only be unlocked, however, if success rates and scales of restoration increase substantially beyond what is currently being achieved around the world. Restoration projects, particularly for mangroves and seagrasses, often struggle to meet their success criteria due to myriad biophysical, socioeconomic and governance constraints. Issues of land tenure and opportunity costs often push mangrove restoration projects into uncontested but suboptimal spaces in the lower intertidal or upper subtidal zone, or seagrasses are restored in areas where the main driver of ecosystem degradation (such as water pollution) has not been addressed. However, guidelines and examples exist to overcome such barriers, and improve the success of coastal habitat restoration and the carbon benefits they provide.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2024.9.
Data availability statement
No primary data were generated for this manuscript.
Acknowledgments
Thank you to T Spencer (University of Cambridge) for the invitation to contribute this review, and N Enright (U.S. Geological Survey) and the Mangrove Lab (Tulane University and National University of Singapore) for comments on a previous draft of this manuscript. Thank you to colleagues of the Blue Carbon Initiative, who have shaped many of the ideas presented here. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author contribution
Conceptualization, visualization; writing – original draft, writing – review and editing: D.A.F.; Writing – original draft, writing – review and editing: Z.I.S.; Writing – original draft, writing – review and editing: M.S.; Visualization, writing – original draft, writing – review and editing: M.M.B.; Writing – original draft, writing – review and editing: C.J.E.L.; Visualization, writing – original draft, writing – review and editing: N.I.
Financial support
D.A.F. thanks Michael and Mathilda Cochran for endowing the Cochran Family Professorship in Earth and Environmental Sciences at Tulane University, which supported this study. NI was supported by a gift from Temasek Holdings.









Comments
Dear Professor Spencer,
Thank you for your kind invitation to contribute a manuscript on ‘Restoring Blue Carbon Ecosystems’ to your journal. Blue carbon is a topic of rapidly increasing importance in coastal conservation, and is being used as a driver of numerous wetland restoration projects. This manuscript brings together a number of predominantly early career researchers who are emerging international experts on this topic, covering the scale of blue carbon restoration available globally, the blue carbon benefits of restoring mangroves, saltmarshes and seagrasses, and some of the current constraints to upscaling the restoration of these important ecosystems.
We think it will be of interest to your multidisciplinary audience, and we welcome the comments of Editors and Reviewers.
With best wishes,
Dan Friess