INTRODUCTION
West Nile fever (WNF) is a mosquito-borne disease caused by a Flavivirus belonging to the Flaviviridae family. The virus is primarily transmitted from bird to bird by mosquitoes, mainly of the Culex genus. Following favourable environmental conditions, this basic cycle may be amplified leading to horse and human infections. The latter two are assumed to be dead-end hosts [Reference Dauphin1]. Due to the involvement of many bird and mosquito species and several potential transmission routes – transovarian transmission in Culex mosquitoes [Reference Dohm, Sardelis and Turell2], bird-to-bird transmission [Reference Hartemink3], persistent infection in birds [Reference Komar4], and transmission by ticks [Reference Anderson5] – WNF epidemiology is complex and multifactorial, and the influence of the environment and climatic context on West Nile virus (WNV) circulation in the USA, Europe, and Africa remains poorly understood. In Europe and Africa, WNV circulation has been recorded in very different ecosystems and climatic contexts including wetlands (Camargue, France, 2000; Senegal River valley, Senegal, 2003; Volga delta, Russia, 1999) [Reference Durand6–Reference Lvov8], urban areas (Bucharest, Romania, 1996; Dakar, Senegal, 2002–2005) [Reference Savage9, Reference Cabre10], floodplain forest-meadow ecosystem (South Moravia, Czechland, repeated isolations of the virus and five human cases of WNF) [Reference Hubálek11] and dry areas (Var area, France; Ferlo area, Senegal) [Reference Durand12, Reference Chevalier13]. In Europe, the richer biodiversity of birds and the structure of the landscape were suspected to be factors favouring the circulation of WNV [Reference Durand12, Reference Pradier, Leblond and Durand14, Reference Ezenwa15]. In the USA, several environmental or climatic risk factors were identified as positively correlated with higher WNV transmission depending on the study region, including farming activity and high temperatures [Reference Miramontes16], urban/suburban environment (vs. mountainous regions) [Reference Gibbs17], or the presence of vegetation in an urban context [Reference Ruiz18]. A reverse relationship between precipitation and WNF outbreaks was demonstrated in Western and Eastern USA [Reference Landesman19]: drought was shown to be positively correlated with an increased WNV transmission level between birds and mosquitoes by concentrating both populations in smaller flooded areas [Reference Shaman, Day and Stieglitz20].
The Senegal River valley, located in northern Senegal, is composed of a large delta that is known to be a major wintering area for palearctic birds. This valley also includes typical Sahelian areas, cultivated and urban zones. In this region, WNV was isolated several times from Aedes and Culex mosquitoes [Reference Traore-Lamizana21] and a serological survey showed WNV circulation in resident wild birds trapped in the wetland [Reference Chevalier7]. However, WNV transmission to horses has never been assessed in this area.
In 2005, an eco-epidemiological study was carried out in five ecologically different zones of the Senegal River valley. The goals were to (i) assess seroprevalence of anti-WNV antibodies in horses in order to infer epidemiological implications of WNV circulation in the area and (ii) identify environmental factors potentially linked to WNV infection.
METHODOLOGY
Study area
The study area was located in northwest Senegal, lying between 15·72° and 16·65° N and −16·64° and −15·55° E. This area is composed of contrasting landscapes with wet areas (swamps, rice fields) bordering the Senegal River and dry areas (herbaceous savannah, coastal dunes). The region has two climatic seasons: a rainy season from June to October that is characterized by heat, humidity and storms, and a dry season from November to May characterized by the Harmattan, a dry dusty wind that blows along the northwest coast of Africa.
Within this area, five study zones were identified. These zones were chosen as being representative samples of the different land covers in the area based on image interpretation of a Landsat Enhanced Thematic Mapper (ETM+) satellite image (4 March 2003) and field observations (Fig. 1).
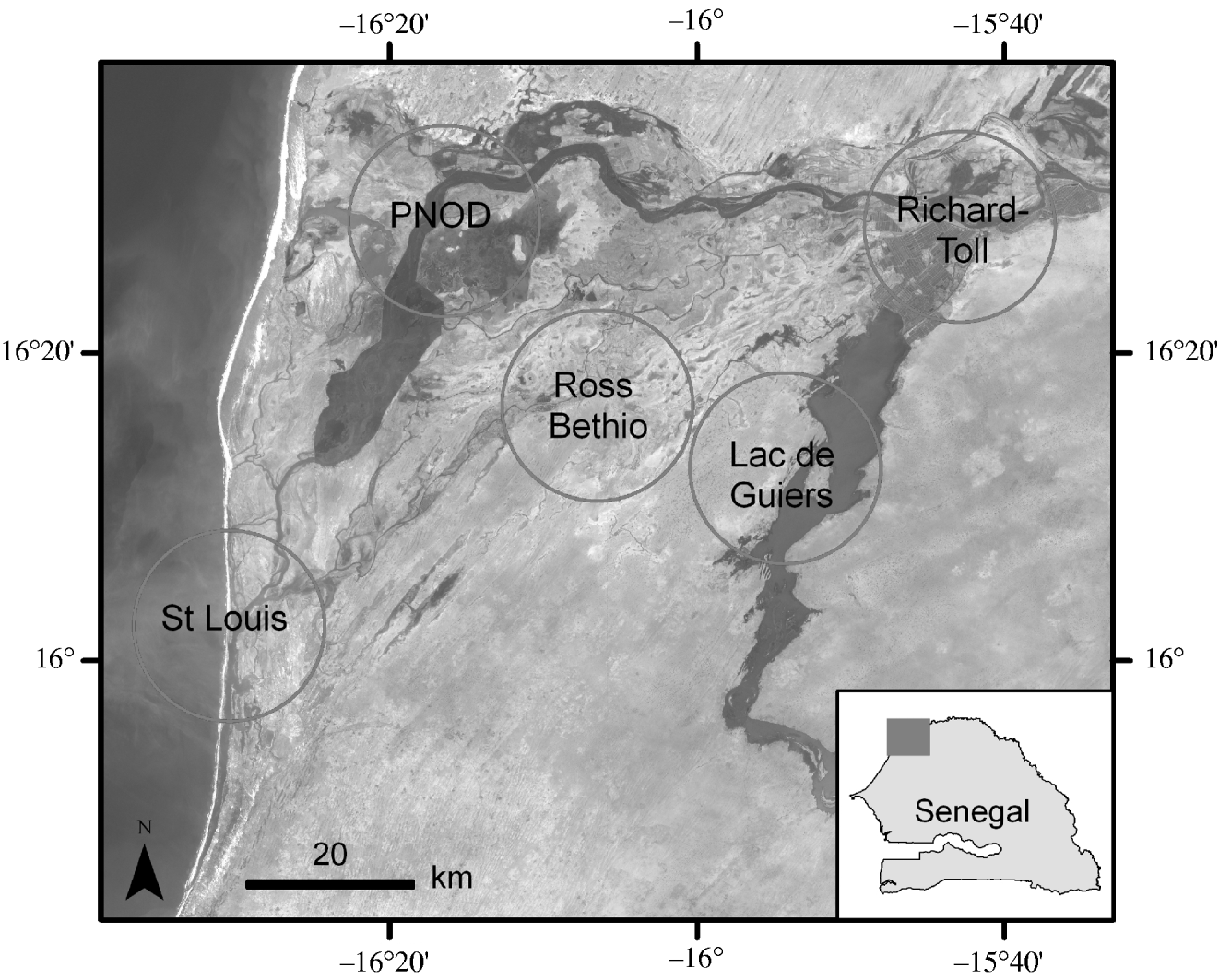
Fig. 1. Study area and location of the field study sites for the serological survey.
The first zone stretched from west to east around the city of St Louis. The heart of St Louis is located on a narrow island lying in the Senegal River. The river is separated from the Atlantic Ocean to the west by a narrow sandy stretch, developed into an urban area. A third part of the city lies on the eastern mainland and is almost surrounded by tidal marches. Around St Louis, the landscape is characterized by occasional acacias and dry savannah. When the river overflows into the countryside, St Louis is surrounded by flood basins. The salt water creates small ponds and stretches of mangroves where many birds come to feed.
The second zone was located in the Djoud'j National Park (PNOD). The PNOD is a 16000-ha wetland located on the border between Mauritania and Senegal in the Senegal River delta. PNOD is a fragmented area composed of a large lake surrounded by streams, ponds, and backwaters. Land cover is mainly composed of acacia swamps, muddy areas, reed bed, and rice fields. The PNOD is known as a major African wintering place for palearctic birds.
The third study site was centred around Ross-Bethio. The landscape surrounding Ross-Bethio is typically Sahelian, characterized by sand dunes and the absence of green vegetation apart from some scattered trees and shrubs during the dry season. During the rainy season the vegetation is composed of an herbaceous layer and a sparse woody plant population.
The fourth study zone was centred around Richard-Toll. Richard-Toll is located near the Senegal River, 80 km from St Louis. In addition to the city, this study site included small fields of crops such as rice and sugar cane and savannah.
The last study site was centred around Nguith village located on the banks of Lac de Guiers, known for its large population of migrating birds. The western part of this site is mainly composed of dry savannah.
Epidemiological and serological data
One market in each of the study zones was selected. Eighty horses then were randomly selected in each of the markets and blood samples taken. The ages and origins of all of the horses were recorded. Whenever possible, the GPS coordinates of where the horses lived were noted. Around each market, a 10 km radius buffer zone was defined. Horses that came from outside this buffer zone were discarded from the study.
Blood samples were centrifuged and stored at 4°C before being systematically tested for WNV-specific IgM by indirect immunocapture of IgM, and for WNV-specific IgG by using antigen capture ELISA. Antigens were prepared from crude brain extracts of WNV-inoculated suckling mice. Serum specimen were considered positive for IgG when the ratio between the optical density (OD) obtained with WNV-positive antigen and WNV-negative antigen was >0·2. Because of the antigenic cross-reactivity in viruses of the Flavivirus genus, and of the circulation of the Usutu virus in Senegal, an antigenically, closely related mosquito-borne member of the genus Flavivirus, validation of ELISA IgG-positive samples was necessary. All the IgG-positive sera were tested against two prototypes: WNV (B956 and Eg101) and Usutu virus (USU-SAAR) in a plaque reduction neutralization test (PRNT). All IgM-negative sera were re-tested using the same methodology. In a 96-well plate, a 1/10 dilution of each serum sample was incubated at 37°C for 1 h in a viral suspension of 20–50 p.f.u. in 60 μl, before the addition of a porcine PS cell suspension (6·104 cells/well). After 4 h incubation, 120 μl L-15 medium containing 0·6% methyl cellulose with 3% fetal bovine serum was overlaid on the well and the plates were incubated at 37°C, in 5% CO2 for 3.5 days. The cells were stained with naphtol Blue Black solution and visualized plaques were counted. A test result was considered positive if the plaque reduction was >90%. Sera that were positive against both WNV and Usutu virus were titrated by twofold dilutions. Dilutions corresponding to 50% reduction of p.f.u. were regarded as the serum titres. At least a fourfold difference of WNV vs. Usutu virus titres was required to assess WNV positivity.
Environmental data
Two satellite ETM+ images from the dry and wet seasons were used to provide a land-cover map of the study area. This type of sensor was chosen because the resolution (pixel size: 30×30 m) is adapted for the extraction of the habitats of birds, hosts of WNV, and because it made it possible to cover the entire study area. To identify the main land-cover types likely to influence the presence and/or composition of the bird population, an ecological characterization of bird habitats was first performed on the entire study area during a field survey conducted in March 2005 by a multi-disciplinary team (ornithologist, geographer, ecologist): 230 sites were georeferenced and described according to the bird population.
A supervised classification was then performed on the dry season image using these sites as a training dataset [image processing software: ERDAS Imagine (Leica Geosystems, USA)]. The second wet season image was processed to map the areas that flooded after the rainfall period. The accuracy of the classification was evaluated using a confusion matrix using a different set of training data acquired in February 2006 (190 ground control points).
From this land-cover map, the surface covered by each land-cover type was calculated for each 10 km radius study zone using Geographic Information System (GIS) functionalities (GIS software: ESRI ArcGISTM, Spatial Analyst, Leica Geosystems GIS Mapping). As the surfaces occupied by the cover types within a buffer were highly correlated, a principal components analysis (PCA) was performed to synthesize the initial information on the landscape into independent factors, i.e. landscape principal component (LPC).
Statistical modelling of prevalence variation
The serological data were analysed using a generalized linear mixed model, with the individual serological status as the binomial response and age as a fixed effect. Since horses were clustered by sites, site was included as a random clustering effect, which also allowed quantifying the inter-site variance.
As a first step, we investigated the effect of age on prevalence. We considered three different models. In the first model, we assumed that prevalence varied linearly as a function of age on a logit scale. In the second model, where the logit of prevalence was linearly related to the log of age, we assumed that prevalence levelled off after a certain age. In the third model, we assumed that prevalence varied in three discrete age classes that were defined as follows: [1, 5), [5, 8), and [8, +). In these models, variation of prevalence in sites was accounted for by a site random effect. These three alternative models for age variation in prevalence were compared using Akaike's Information Criterion (AIC).
For the second step, in order to depict age variation we included, in the selected model, the fixed effect of each of the principal landscape components which were likely to underlie the variation in prevalence in sites.
RESULTS
A total of 367 horses were included in the study, their age ranging from 2 to 24 years (Fig. 2). The overall IgG seroprevalence rate was 85% (95% CI 0·81–0·89). The prevalence rates varied significantly from one study site to another (χ2=16·17, d.f.=4, P=0·003), with the highest prevalence in the Nguith site (P=95%), followed by Richard-Toll (P=92%), Ross-Bethio (P=86%), and PNOD (P=82%). The lowest prevalence rate was observed in the St Louis site (P=74%) (Table 1). No IgM antibodies were found in the samples tested.
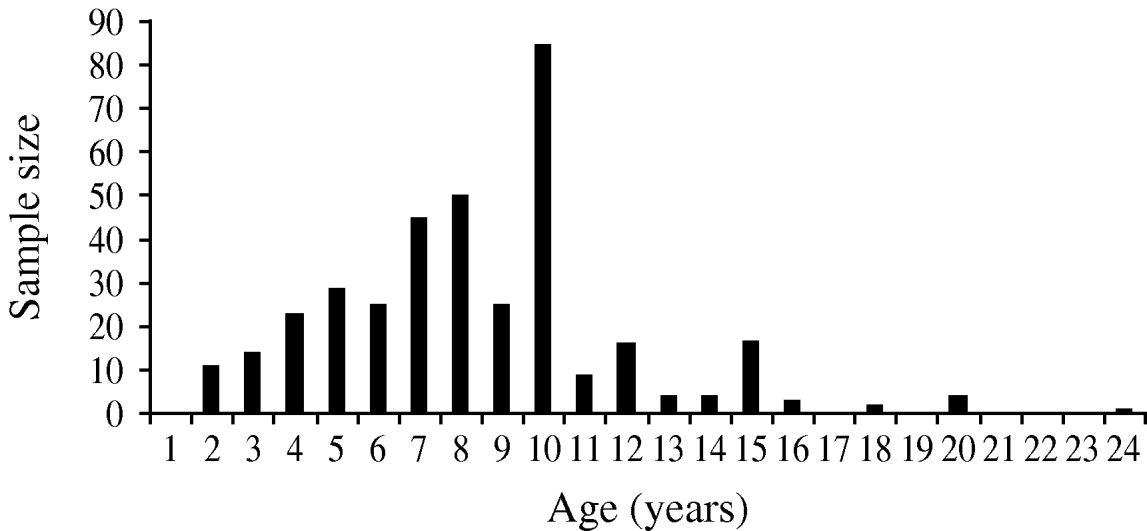
Fig. 2. Age structure of the horse samples (n=376) studied to estimate the serological prevalence of West Nile virus infection in the Senegal River basin in 2005.
Table 1. Serological prevalence of West Nile virus infection in horses of five contrasted areas of the Senegal River basin in 2005 and landscape principal component (LPC) values
The landscape map derived from the satellite imagery includes 23 classes that belong to four main landscape types: human and cultivated areas, arid land areas, wetlands, and coastal areas (Table 2). The overall accuracy of the classification estimated was correct (82·54%) with 0·79 for the kappa index.
Table 2. Landscape classes (n=23) derived from the satellite imagery and included in the classification
Four synthetic variables (LPC1–4) resulted from PCA. The main positive contributors to LPC1 were sea water, and flooded banks and salted mudflats (known as ‘tans’). The main negative contributors were grassy vegetation and dry tans. LPC2 was mainly composed of halophyte vegetation. LPC3 was negatively composed of free water and LPC4 positively with sugar cane and negatively with never flooded, naked ‘tan’ (Fig. 3).
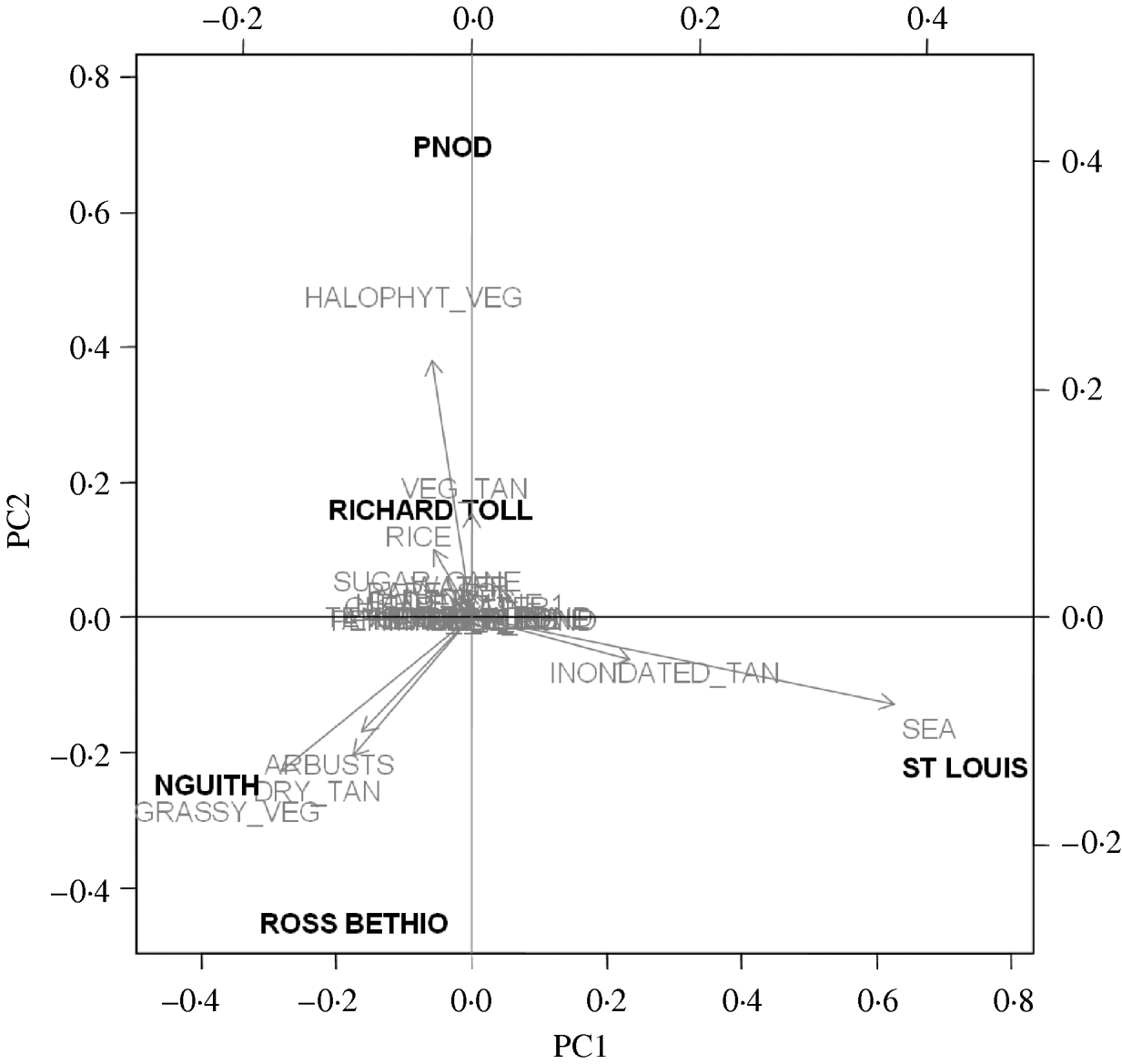
Fig. 3. Composition of the principal components (LPC1, LPC2) and projection of the five study sites on the first principal components analysis plan.
Of the candidate models depicting age variation in prevalence, the best model according to AIC was that where prevalence levelled off after a certain age (Table 3). Furthermore, the model without any age effect performed poorly compared to all of the models including an age effect (difference in AIC was always >13·6). It thus can be concluded that age had a significant positive effect on prevalence (P<0·001) (Fig. 4). The logit of prevalence varied significantly between sites [estimated standard deviation in sites: 0·51 (s.e.=0·23)]. Prevalence was thus linked not only with age but also with site ecological components. LPC1 explained a significant fraction of the variation in prevalence in sites (P=1·5×10−4) suggesting that the components of LPC1 – sea water and tan – were protecting factors against WNV transmission (Table 4) as shown in Figure 5.
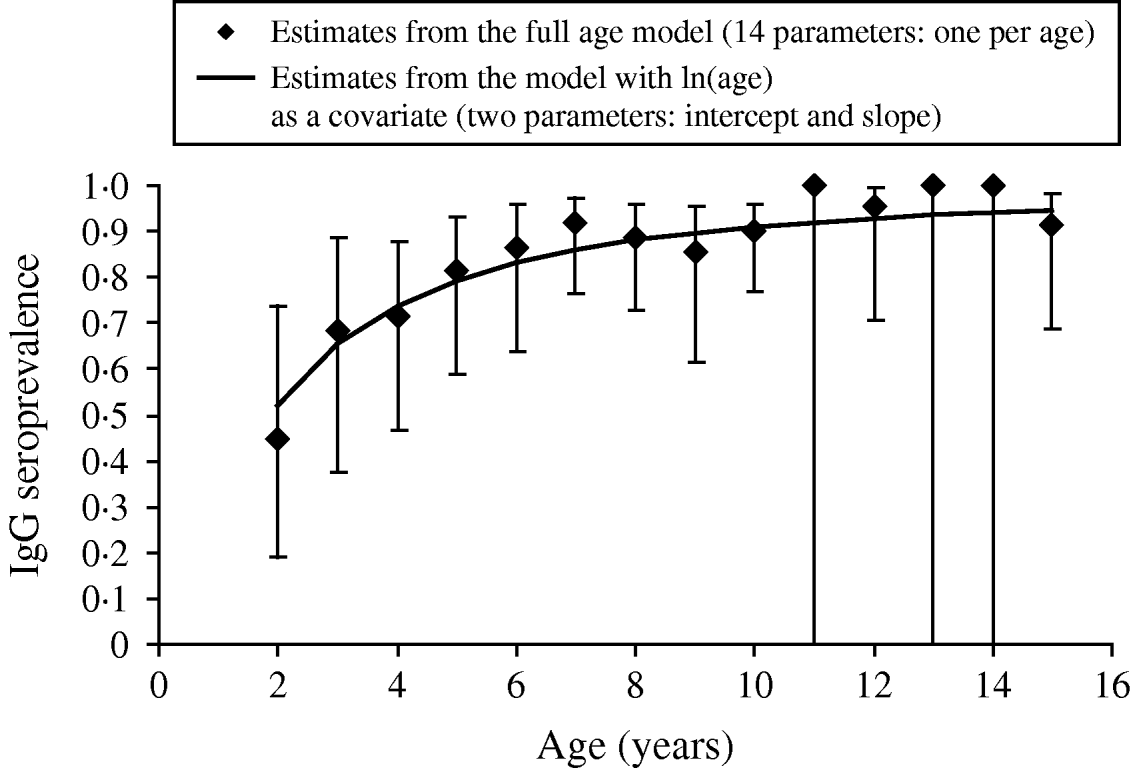
Fig. 4. Age variation in anti-West Nile virus IgG seroprevalence in 79 horses sampled in the PNOD study site (Senegal, 2005). Note that due to additivity of age and site in the model used to depict age variation in prevalence, patterns of age variation in the other sites are similar to that shown here for the PNOD study site.
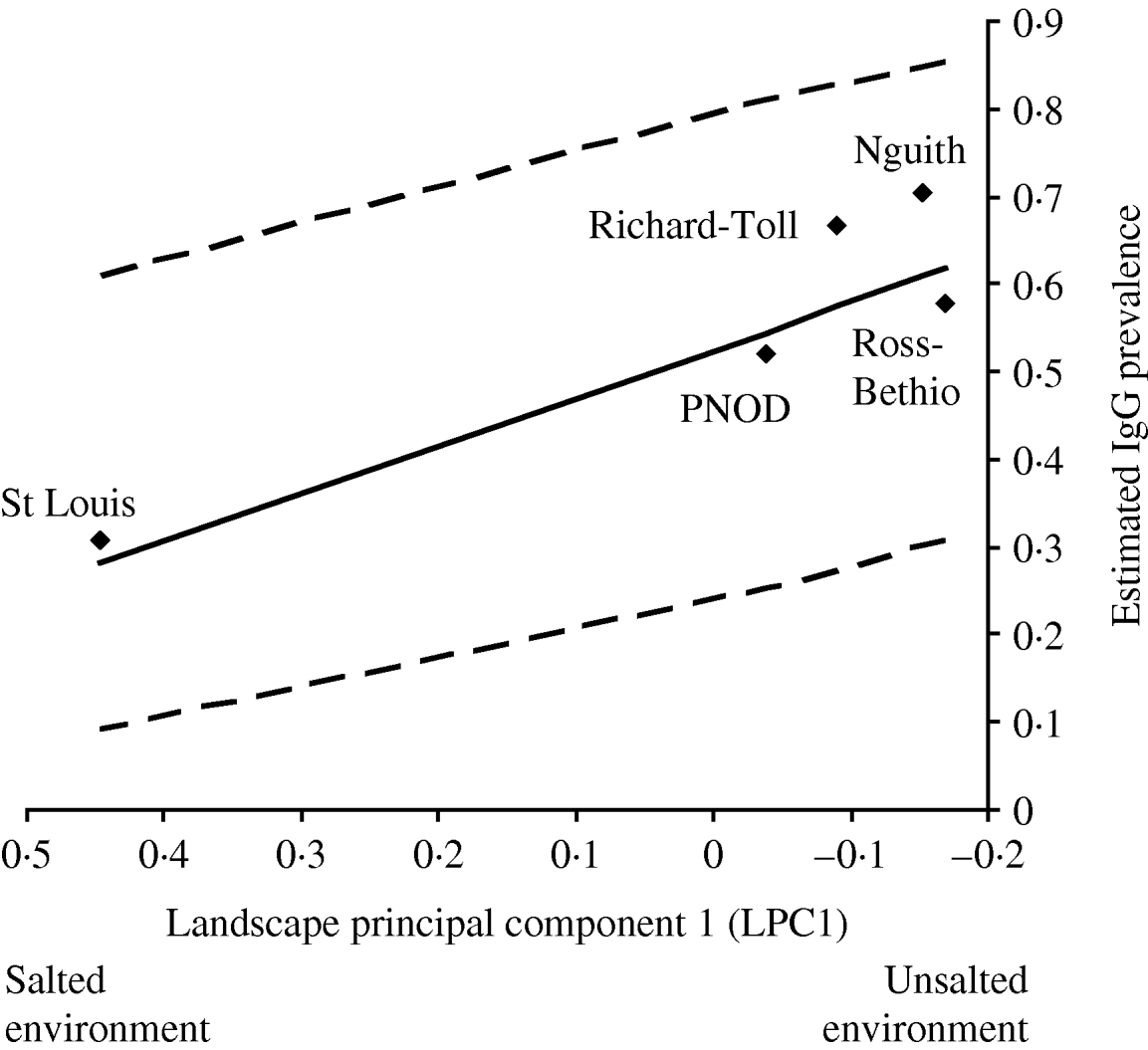
Fig. 5. Prevalence of 2-year-old horses predicted from a model with additive effects of ln(age) and of site (◆), as a function of PC1 values (interpreted as a gradient of saltiness of the habitat). The solid line is the regression line estimated from a model with additive effects of age and PC1. The dashed lines are the 95% confidence intervals of this regression line.
Table 3. Selection of the best model for age variation using Akaike's Information Criterion (AIC)
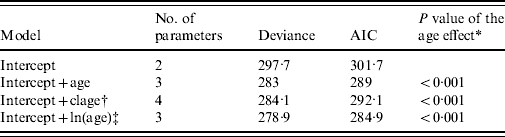
All models include site as a random effect.
† Age classes.
‡ ln of age.
* P values were computed using likelihood ratio tests.
Table 4. Estimates of the parameters included in the models addressing the variation of prevalence with landscape principal components (LPC)
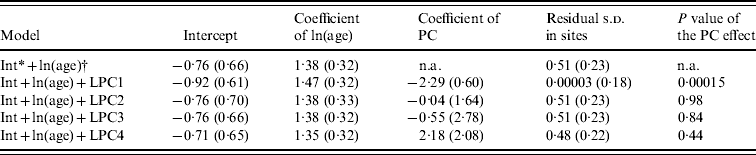
Standard errors are in parentheses.
* Intercept.
† ln of age.
DISCUSSION
The PRNT is considered the gold standard for Flavivirus serology, and is used for validation of other tests [Reference Hubalek22]. However, even PRNT against WNV may lead to false-positive results due to cross-reactivity with other flaviviruses, especially with Usutu virus [Reference Niedrig23, Reference Crill and Chang24]. To eliminate the possibility of cross-reaction with Usutu virus, we tested positive sera against two prototypes: WNV (B956 and Eg101) and Usutu virus (USU-SAAR) using PRNT, a fourfold difference of WNV vs. Usutu titres being required to assess WNV positivity, which is a stringent interpretation criteria.
This study confirms that WNV circulation is endemic in the Senegal River basin as shown by the increase of prevalence with age.
Although the tests were performed in very different ecological contexts, the serological results were in accord with a previous study in the Ferlo area (northern Senegal) and Dakar (Senegal) in which the IgG seroprevalence rates on horses were estimated to be 78·3% (n=120) and 92% (n=25), respectively [Reference Cabre10, Reference Chevalier13]. Considering that horses live in close proximity to humans, the infection rate of horses may be regarded as a good indicator of human exposure. This very high level of transmission would justify the implementation of a surveillance system for WNV circulation in order to anticipate viral mutation that could lead to increased virulence with severe consequences on horse and human health.
IgM seroprevalence, which is an indicator of recent infection, was null: horses were sampled in July when the rainy season had just begun. In the Senegal River basin, the Culex population is somewhat reduced from November to June (B. Mondet, personal communication), starting to increase after the first rainfall events at the end of July. At the beginning of July, the transmission pressure was probably very low, explaining the absence of IgM in the samples.
The location where the horses lived was taken into account for environmental analysis because Culex mosquitoes have a crepuscular or nocturnal activity. We assumed that the location in which the horses lived was also the place where they became infected. Based on expert opinions and previously acquired field knowledge, we assumed that people walk no farther than 10 km from their house to reach the market. This estimation was used to define the radius of the area from which environmental data were extracted under the assumption that horses located in this buffer zone were equally exposed to mosquito bites.
Despite the intense WNV circulation, transmission levels varied according to the study site and landscape type, and statistical analysis suggests that sea water and tan may be protecting factors against WNV transmission in this area. This result is based on data obtained from a single sampling season. However, besides environmental characteristics (resulting from PCA), the model includes equid age, the dependent variable being age- and site-specific seroprevalence. In old animals, this variable covers both recent and past exposure to WNV infection. Furthermore, land-cover types chosen for image classification represent perennial structures. We thus believe that the environmental risk factors we obtained are valid not only for the study year but also for past years.
C. pipiens and C. modestus are the main vectors of WNV in Europe [Reference Hubalek25]. However, there is lack of knowledge on WNV mosquito vectors – identity, distribution and population dynamic, in the studied area. Several observations or studies suggest that C. poicilipes may be one of the major WNV vectors: (i) this mosquito species is one of the most abundant species in the Senegal River basin [Reference Diallo26, Reference Fontenille27], probably because of well developed rice plants as well as the presence of a varied community of aquatic plants and animals [Reference Snow28]; (ii) this species is predominantly ornithophilic [Reference Gordon29], and (iii) WNV was isolated from this mosquito species in Senegal (CRORA database http://www.pasteur.fr/recherche/banques/CRORA). This species was also mentioned as a WNV carrier in Egypt [Reference Orshan30]. The biology of Culex mosquitoes, may explain the results of the environmental analysis. It is known that Culex mosquitoes prefer water contaminated with organic matter or slightly brackish, rice fields, irrigation canals, and semi-permanent marshes for the development of larvae [Reference Beaty and Marquardt31]. Urban and cultivated areas are thus favourable habitats for this mosquito species. The same may not be said for ‘tan’, which are very dry and sometimes salty surfaces where only acacias can grow. This land-cover type is thus particularly unfavourable to these mosquitoes, as is salty water, in which Culex larvae can not survive. Moreover, the wind blowing in from the sea may explain why WNV transmission is lower in St Louis than in the other selected sites. As the two identified protecting factors against WNV transmission are unfavourable factors for Culex mosquitoes, our results suggest that in Senegal, the distribution of the vector species is more limiting for WNV transmission than for the hosts' distribution.
In Europe, many outbreaks occurred in salty environments or wetlands located along the sea coast, suggesting that observations performed in Africa cannot be extrapolated to Europe. Similarly, the highest seroprevalence rate was detected in Nguith, and not as expected in PNOD, because of its high level of landscape fragmentation [Reference Pradier, Leblond and Durand32]. West African and European WNV epidemiological systems should be distinguished from each other; the identification through an environmental approach of factors favouring WNV circulation in Africa consequently cannot be transposed to Europe. Long-term longitudinal studies in sentinel domestic and wild birds as well as small-scale environmental studies may help identify these factors.
Finally, the Senegal River basin is known as a major wintering area for European migrating birds. As previously suggested [Reference Zeller and Schuffenecker33, Reference Malkinson and Banet34] this could transport the virus to Europe and contribute to the initiation of WNV circulation in temperate regions. Further investigative studies are needed on the role of migrating birds in the transport and emergence of WNV in Europe and on the factors involved in this process in order to build a prediction model, and test the effect of climate and meteorological events, migration patterns, local conditions, and the role of nestling birds in cycle amplification.
ACKNOWLEDGEMENTS
This study was partially funded by the CORUS project (French Ministry of Foreign Affairs) and GOCE-2003-010284 EDEN grants. This publication is catalogued by the EDEN Steering Committee as EDEN00xx (www.edenfp6project.net). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
DECLARATION OF INTEREST
None.













