Introduction
One hundred sixty-five million episodes of shigellosis are estimated to occur annually worldwide, and the vast majority of cases and deaths occur in low- to middle-income countries in children under 5 years of age [Reference Kotloff1]. The Global Enteric Multicentre Study, a matched case–control study of moderate-to-severe diarrhoea in children aged 0–24 months, showed that shigella is among the top ranking pathogens in the South American countries (Peru and Brazil) [Reference Platts-Mills2]. The shigella genus is composed of four serogroups (formerly known as species), i.e. Shigella flexneri, Shigella dysenteriae, Shigella boydii and Shigella sonnei, which are the aetiological agents of bacillary dysentery and shigellosis. A uniquely small inoculum of shigella (as few as 10 organisms) is required to cause dysentery, which facilitates person-to-person transmission [Reference Anderson, Sansonetti and Marteyn3].
High rates of resistance to conventional antibiotics such as ampicillin, chloramphenicol and cotrimoxazole have been described in many studies [Reference Niyogi4]. Thus, extended-spectrum cephalosporins, fluoroquinolones and azithromycin have come into use as front-line antibiotics. The clinical severity of shigellosis and the emergence of resistance against first-line therapies highlight the growing need to develop a vaccine against shigella infections. However, the existence of four different serogroups and at least 50 antigenically distinct serotypes is a barrier. Indeed, the larger the number of serotypes to be included, the more complex and expensive the vaccine becomes. As the serotype distribution differs by geographical region, a better understanding of the serotype distribution of shigella species at a national level is important in order to inform vaccine development [Reference Livio5].
French Guiana is a French overseas department located in South America. The subequatorial climate is characterised by a dry season (from the end of July to mid-November) alternating with a rainy season (from mid-November to the end of July), which is sometimes interrupted by a small dry season in March. The majority of the population lives on the coast (90%), and 10% of the population lives along the rivers – especially the Maroni River and the Oyapock River – or far inland. Some people have difficulty accessing drinking water. In 2010, almost 15% of the population did not have drinking water at home, including 70% of people living in rural areas. These drinking-water access problems are responsible for the spread of waterborne pathologies – such as typhoid fever – which cause epidemics, and can even become endemic [Reference Mansotte, Margueron and Maison6].
There are few data available on shigellosis in French Guiana. A retrospective study was conducted in Saint-Laurent du Maroni's Hospital between 2000 and 2014 among pregnant women (37 cases of shigellosis diagnosed). S. flexneri and S. sonnei were the two species isolated from maternal stools [Reference Parisot7].
This article aims to describe the strains of shigella isolated in children under 5 years of age (prevalence, distribution of species and serotypes, antimicrobial resistance), to identify seasonality in case distribution and to estimate the incidence of disease.
Materials and methods
Study population
A retrospective study was carried out of all shigella strains isolated between 2000 and 2012 from stools, urines, gastric samples and blood in children under 5 years of age at the bacteriological laboratory of Saint-Laurent du Maroni Hospital. This hospital is the second hospital in French Guiana and the only secondary health care facility in western French Guiana. The catchment area of this hospital included 79 500 inhabitants of which 51% lived in Saint-Laurent du Maroni the main town and 27% in rural areas in inland. The population is young and multi-ethnic. Children under 5 years of age accounted for 18% in 2010 [8].
Data collection
We collected from the laboratory dataset: demographics data (age, gender).
Bacterial strains, serotyping and antibiotics susceptibility of shigella strains
Shigella was identified by conventional methods and serotyping was performed by slide agglutination assays with a complete set of antisera recognizing all the described shigella serotypes [Reference Langendorf9]. The susceptibility of the shigella isolates to antibiotics was assessed using the VITEK 2 Compact system in accordance with the recommendations of the European Committee on Antimicrobial Susceptibility Testing [10]. The antibiotics tested were: cotrimoxazole, amoxicillin/clavulanic acid, amoxicillin, ampicillin, ticarcillin, piperacillin/tazobactam, cefalotin, cefotaxim, ceftazidim, cefoxitin, imipenem, tobramycin, gentamycin, amikacin, netilmycin, nalidixic acid, ofloxacin and ciprofloxacin. If more than one isolate with the same serotype and antimicrobial resistance phenotype was recovered from the same patient, only the first was included.
Statistical analysis
The number of cases every month was compared with the average monthly precipitation between 2002 and 2012. The data were obtained from the Météo France website [11].
The incidence rate between 2006 and 2012 was estimated from a census of children under 5 years of age in western French Guiana during the same period.
A series of inferential statistics was conducted to compare antimicrobial resistance across shigella serogroups or time periods, using χ 2 test, Yates corrections or Fisher's exact test applied as needed. Spearman's coefficient correlation was used to demonstrate a correlation between the number of cases each month and average monthly precipitation. The α level was set at 5%.
The study was carried out with the approval of the Saint-Laurent du Maroni hospital ethics committee.
Results
Prevalence, serogroups and serotypes
Shigella was the second bacterial agent most frequently isolated, identified in 3366 stool samples (6.2%), after the enteropathogen Escherichia coli (8.3%). The other genera of bacteria isolated were Salmonella sp (3.6%), Campylobacter sp (3.1%) and Yersinia sp (0.01%).
In total, 213 strains of shigella were isolated between 2000 and 2012 from 213 infants and children under the age of 5 years with diarrhoea: 210 strains were isolated from stool samples, two from urine and one from a gastric sample. No strains were isolated from blood.
Male accounted for 51% of the patients (n = 109). The median age was 1.1 years, the 25th percentile was 0.7 years and the 75th percentile was 2.1 years. Children under 1 year of age accounted for 45% of the cases, including 15% of children under 6 months. Three neonatal cases defined by the presence of shigella in stool in children under the age of 1 month were observed.
The serogroup was determined for 210 strains of shigella, including 161 (77%) S. flexneri strains, 48 S. sonnei strains (23%) and one S. boydii strain. The indeterminate strains are referred to as Shigella spp hereafter. No S. dysenteriae strains were isolated.
Eight serotypes of S. flexneri were identified. The four major serotypes were serotype 2a (n = 100, 62%), serotype 3a (n = 26, 16%), serotype 1b (n = 12, 7%) and serotype 6 Boyd 88 (n = 9, 6%). The other serotypes were serotype 3b (n = 2, 2%), X, Y and 1a (n = 1, 1% each one). The serotype for nine S. flexneri strains was indeterminate. Serotype 2a was predominant throughout the study period. All S. sonnei strains belonged to biotype g. The biotype for two S. sonnei strains was indeterminate. The S. boydii strain belonged to serotype 20.
Antimicrobial resistance
The antimicrobial resistance is summarised in Table 1. The resistance profiles of S. flexneri and S. sonnei differed. Significantly more S. sonnei strains than S. flexneri strains were resistant to cotrimoxazole (96% vs. 69%, P < 0.001). A higher proportion of S. flexneri strains than S. sonnei strains were resistant to ampicillin (73% vs. 10%, P < 0.001), amoxicillin (37% vs. 0%, P < 0.001), ticarcillin (74% vs. 6%, P < 0.001) and cefalotin (17% vs. 4%, P = 0.03). The S. boydii strain was resistant to amoxicillin and ticarcillin, but was sensitive to cotrimoxazole. All of the strains were sensitive to aminoglycoside, fluoroquinolone and third-generation cephalosporins. One S. sonnei strain was resistant to nalidixic acid.
Table 1. Antimicrobial resistance in Shigella flexneri, Shigella sonnei and Shigella spp strains

The results are expressed in number of resistant strains/number of strains tested and then in per cent.
*The P-value referenced the comparison between S. flexneri and S. sonnei strains.
The changes in resistant strains of S. flexneri and S. sonnei during 2000–2004, 2005–2008 and 2009–2012 are summarised in Tables 2 and 3. Among the S. flexneri strains, the resistance to clavulanic acid/amoxicillin increased, reaching 30% of strains between 2009 and 2012 (P < 0.001). The resistance to cotrimoxazole dropped between 2005–2008 and 2009–2012 (from 93% to 54%, respectively, P < 0.001). The resistance to ampicillin and ticarcillin decreased, but the difference was not significant. Among the S. sonnei strains, the resistance to cotrimoxazole increased but the difference was not significant. All the S. sonnei strains tested from the 2009–2012 period were resistant to cotrimoxazole. Few strains were resistant to β-lactam antibiotics, and only 8% of strains tested from 2009 to 2012 were resistant to ampicillin and ticarcillin.
Table 2. Evolution of the antimicrobial resistance in Shigella flexneri strains between 2000 and 2012

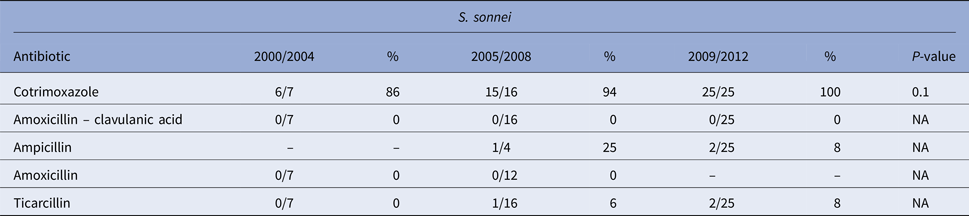
Table 3. Evolution of the antimicrobial resistance in Shigella sonnei strains between 2000 and 2012
Seasonality
The relationship between average monthly precipitation and the number of cases per month is shown in Figure 1. An inverse relation was observed. More strains were isolated during the warmer and dryer months of the year (July to December). The correlation was high and significant (Spearman's coefficient correlation = −0.8, P < 0.001).
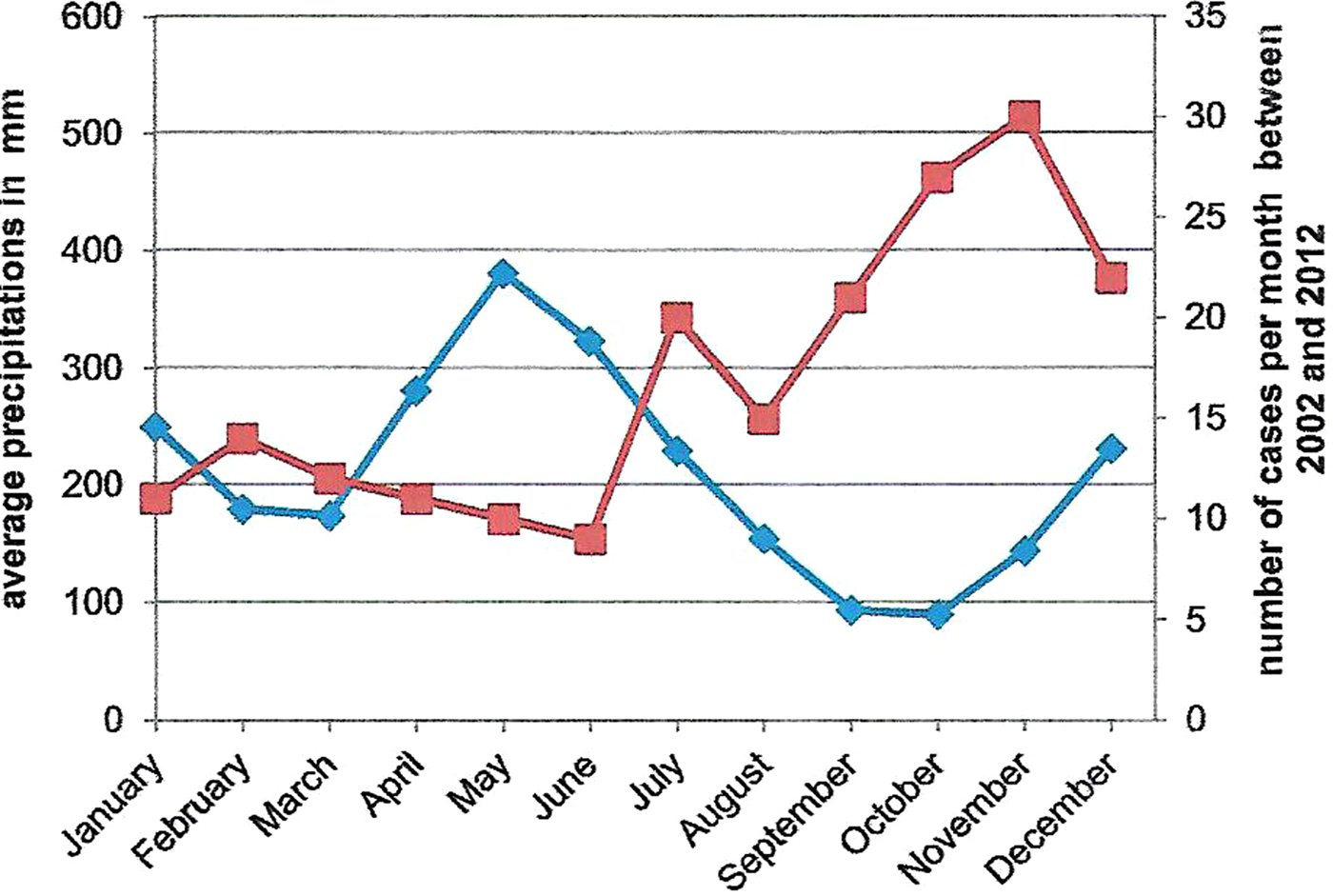
Fig. 1. Comparison between average monthly precipitations (bleu curve) and the number of cases per month from 2002 to 2012 (red curve) in Saint-Laurent du Maroni.
Incidence
The incidence in western French Guiana in children under 5 years of age varied between 112.6 and 277.3 cases per 100 000 inhabitants between 2006 and 2012. The average incidence was 189.6 cases per 100 000 inhabitants.
Discussion
This is the first study of shigellosis in children under 5 years of age in western French Guiana. This is the population in which the morbidity is the most significant [Reference Kotloff1]. The incidence is underestimated for at least two reasons. First, shigella is fragile, which makes it difficult to isolate this bacterium. Stool samples need to be inoculated within half an hour following their collection, and specific storage conditions are needed. The bacteriological examination can be negative even when the sample contains bacteria [Reference Breurec12]. Second, cases that occur in the isolated areas along the Maroni River are not accounted for. Because these areas are only accessible by boat or plane, transporting samples under the proper conditions is difficult.
The predominance of S. sonnei over other serogroups is observed when economic conditions improve and the level of sanitation increases. The frequency of isolation is directly correlated with the gross domestic product per capita [Reference Ram and Part13]. The clear predominance of S. flexneri in western French Guiana reveals the presence of unsanitary conditions. This finding is consistent with the known distribution of shigella serogroups in countries with a low socio-economic level [Reference Kotloff1].
To the contrary of serotypes in S. dysenteriae (15 serotypes) and S. boydii (20 serotypes), major cross-reactions were observed in animal experiments for 14 of the 19 serotypes of S. flexneri, due to a degree of antigenic relatedness attributable to a common repeating tetrasaccharide unit [Reference Levine14]. Thus, a multivalent vaccine including O antigens from S. flexneri 2a and 3a, in addition to direct protection against S. flexneri 2b and 3b, would provide cross-protection against S. flexneri 1a, 1b, 4a, 4b, 5a, 5b, 7b, X and Y [Reference Levine15, Reference Noriega16].
Extrapolating these data to humans, a quadrivalent vaccine including S. sonnei (only one serotype described), S. flexneri 2a, 3a and 6 would provide broad coverage against shigella, through direct (87%) and cross-protection (94%). This level is in agreement with the rate observed in two multicentre studies at four sites in Africa and nine sites at Asia, in which a quadrivalent vaccine including the serotypes listed above would have provided protection against at least 85% of the serotyped strains [Reference Livio5, Reference Von Seidlein17]. Although these cross-reactions remain theoretical and discrepancies exist between data for humans and animals [Reference Farzam18], a quadrivalent vaccine containing a limited number of serogroups and serotypes could provide broad coverage against shigella.
In Thailand, the incidence in the overall population and among children under 5 years of age is estimated at 600 and 4000 cases per 100 000 inhabitants per year, respectively [Reference Chompook19]. In Kenya, the incidence in the overall population is estimated at 408 cases per 100 000 inhabitants per year, and ranges from 136 to 369 cases per 100 000 inhabitants per year among children under 5 years of age [Reference Njuguna20]. In mainland France, the infection spreads in an epidemic pattern. Epidemics often occur in communities and involve young children. The main serogroup isolated is S. sonnei, and the incidence is low, on the order of 1.8 cases per 100 000 inhabitants per year [21]. In Canada and Belgium, the incidences are estimated at 1.9 and 3.7 cases per 100 000 inhabitants per year, respectively, in the overall population [Reference Vrbova22, Reference Bertrand23]. Our data suggest that western French Guiana's incidence in children under 5 years of age is closer to low- than high-resource settings.
The shigella strains were resistant to antibiotics that are no longer recommended for treating shigellosis. All of the strains were sensitive to fluoroquinolones and third-generation cephalosporin. The sensitivity to azithromycin was not determined. The same resistance profiles have been observed in strains isolated in eastern and northern Brazil [Reference Bastos and Loureiro24].
Individuals carrying enterobacteria resistant to third-generation cephalosporin have been reported in French Guiana: a study carried out in Trois Sauts – an Amerindian village in a protected area – estimated that 8% of healthy adults carried resistant enterobacteria. Resistance to third-generation cephalosporin was only observed in E. coli [Reference Woerther25]. These strains carried heterogenic CTX-M type β-lactamases, of which 4.3% were CTX-M2 type. These data indicate that resistant strains circulate even in remote corners of French Guiana. The emergence of shigella or salmonella strains that are resistant to third-generation cephalosporin could occur due to the acquisition of plasmids that encode β-lactamase from the intestinal flora.
Breastfeeding decreases the severity of the disease and protects the host against shigella invasion. This protective effect seems to be related to the transmission of immunoglobulin A (IgA) antibodies and the action of lactoferrin – an antimicrobial glycoprotein that is present in breast milk and in most human mucosal secretions [Reference Gomez26, Reference Willer, Lima and Giugliano27]. Both IgA and lactoferrin seem to be bind to superficial proteins involved in the mechanism of the cell invasion. The significant prevalence of shigellosis in children under 1-year old in this study (45%) could be due to insufficient breastfeeding in this population, and could highlight a lack of hygiene in preparing baby bottles or the use of contaminated water. This hypothesis is supported by data from a survey on breastfeeding conducted between 2010 and 2012 among 96 women from the pregnancy network ‘Périnat Guyane’ (data not published). In the maternity ward, the rate of breastfeeding was estimated at 94%. However, the rate decreased rapidly after the first month, reaching 43% at 3 months and 32% at 6 months. The partial breastfeeding rate increased over time, reaching 46% at 6 months.
The seasonal peak of infection in the intertropical zone coincides with the warmer months. In Asia and Brazil, it corresponds with the rainy season [Reference Chompook19, Reference Ângela Bernardes Sousa28–Reference Huang30]. In Turkey, most cases are observed during the dry season [Reference Özmert31]. Several factors could explain the seasonality in western French Guiana. Nearly half of the population that lives near the Maroni River does not have access to safe water, and uses rain water or surface water as a source of drinking water. During the warmer and dryer months, the storage tanks are often empty, and the population has little choice but to obtain water from the river. Between 1995 and 2007, 13 outbreaks of typhoid fever were identified. In most cases, people did not have access to the public supply system and used unsafe water (water from the river in rural areas and rain water in urban areas) [Reference Mansotte, Ravachol and Ardillon32].
Conclusion
Shigellosis is a frequent cause of diarrhoea among children under 5 years of age, especially among children under 1 year of age. Similar to other developing countries, the main species isolated was S. flexneri, and serotype 2a was highly represented.
The seasonal peak of shigellosis coincided with the warm and dry season. These infections suggest safe water source and proper sanitation may be difficult for this population. The high incidence rate should alert public authorities to the need for immediate action to improve water safety.
This study confirms the usefulness of fluoroquinolones and third-generation cephalosporin antibiotics in the treatment of shigellosis. However, continuous surveillance of circulating strains is important for adapting empiric treatment and preventing the emergence of antimicrobial resistance.
Acknowledgements
The study was carried out with the approval of the local ethics committee of Saint-Laurent du Maroni Hospital.
Declaration of interest
None.









