Article contents
Mutagenesis in Escherichia coli
III. Requirement for DNA synthesis in mutation by gamma rays of T4-phage complexed with Escherichia coli
Published online by Cambridge University Press: 14 April 2009
Summary
A system has been developed for the study of reversion of an amber mutation responsible for a deficiency in DNA synthesis in T4 phage E51. When complexed with bacteria able to suppress the amber mutation the induced mutation rate per phage genome per rad is
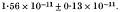
When complexed with bacteria unable to suppress the amber mutation (and being thus unable to synthesize phage DNA) the induced mutation rate is at least 14 times lower indicating that DNA synthesis is necessary for the production of the majority of functional reversions at the amber site. The induced mutation rate in suppressor-containing bacteria is independent of multiplicity of infection between 0·2 and 5, suggesting that recombination immediately after irradiation between phage genomes is unlikely to be a requirement for the mutation process.
- Type
- Research Article
- Information
- Copyright
- Copyright © Cambridge University Press 1970
References
REFERENCES
- 7
- Cited by




