No CrossRef data available.
Article contents
Fibrin and Transforming Growth Factor Alpha Affect Prostatic Smooth Muscle Cell's Phenotype and Motility
Published online by Cambridge University Press: 11 March 2021
Abstract
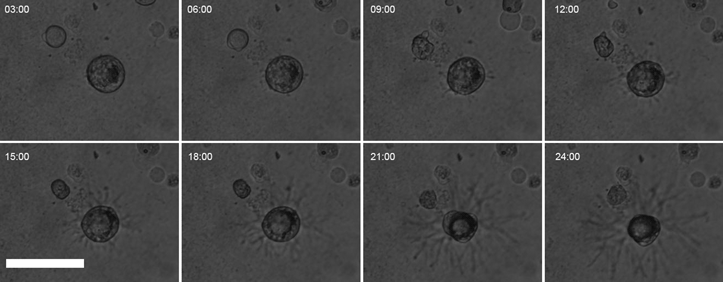
Smooth muscle cells (SMCs) are dynamic and transition from a contractile to a synthetic phenotype under different circumstances. Plasma factors (fibrin and transforming growth factors, TGFs) are possible components affecting SMCs differentiation and behavior. Thus, the objective of this work was to investigate how the fibrin matrix and TGFs affect SMCs differentiation and motility behavior. SMCs invaded the fibrin gel and adopted a stellate phenotype while reducing the expression of differentiation markers (Acta2, Myh11, and Smtn). At the ultrastructural level, SMCs did not assemble a basal lamina and showed numerous blebs along the entire cell surface. This transition was not associated with changes in focal adhesion kinase (FAK) content and phosphorylation status but reflected a marked change in FAK distribution in the cytoplasm. After 48 h in culture, SMCs caused an active degradation of the fibrin gel. Additionally, we tested the SMCs response to TGFs in a cell layer wound repair assay. TGFα, but not TGFβ1 or TGFβ3, had significantly increased motility. In conclusion, prostatic SMCs present a phenotypical transition when cultured on fibrin, adopting a micro-blebbing based motility behavior and increasing migration in response to TGFα.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America





