No CrossRef data available.
Article contents
Low-Voltage Electron-Probe Microanalysis of Uranium
Published online by Cambridge University Press: 18 March 2021
Abstract
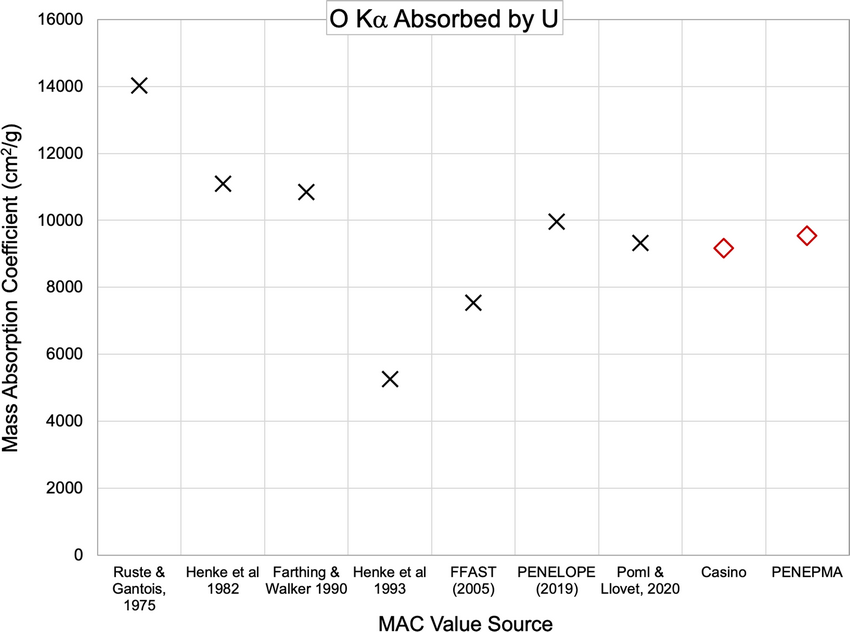
Electron-probe microanalysis of uranium and uranium alloys poses several problems, such as rapid oxidation, large poorly constrained correction factors, and a large number of characteristic x-ray lines. We show that U-metal can grow 10 nm of oxide within ~20 s of air exposure, increasing to 15–20 nm within a few minutes, which can produce a 30% quantification error at 5 kV. A 15 nm carbon coating on the UO2 reference material also produces an ~30% quantification error of the uncoated but surface oxidized U sample at 5 kV. Correcting for both the coating and oxide improved the analysis accuracy to better than ±1% down to 7 kV and ~2% at 5 kV, but the error increases strongly below this. The measurement of C in U identified a previously unreported U N6–O4 line interference on the C Kα peak, which can produce over 1% error in the analysis total. Oxide stoichiometry was demonstrated to have only a small impact on quantification. The measurement of the O Kα and U Mα mass absorption coefficients in U as 9,528 and 798 cm2/g, respectively, shows good agreement with recently published values and also produces small differences in a quantification error.
- Type
- Materials Science Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America





