Introduction
The female reproductive system in insects consists of a pair of ovaries which consist of ovarioles, a pair of lateral oviducts, and a genital chamber. Each ovary continues with a canal called a lateral oviduct where the mature egg moves forward to be left out and the lateral oviducts come together to form a common oviduct of ectodermal origin (Viscuso et al., Reference Viscuso, Narcisi and Sottile1999; Lange, Reference Lange2009; Klowden, Reference Klowden2013; Pappalardo et al., Reference Pappalardo, D'Urso, Viscuso, Ferrrito, Giunta, Cupani and Vitale2016). Besides all these main reproductive organs such as spermatheca (receptaculum seminis) and reproductive glands, there are some other auxiliary structures that help reproduction in female insects which play a role in transfer and protection of the gametes (Nandchahal, Reference Nandchahal1972; Giuffrida et al., Reference Giuffrida, Focarelli, Lampariello, Thole and Rosati1996; Sturm & Pohlhammer, Reference Sturm and Pohlhammer2000; Lange, Reference Lange2009; Brundo et al., Reference Brundo, Longo, Sottile, Trovato, Vitale and Viscuso2011; Sturm, Reference Sturm2012; Masci et al., Reference Masci, Di Luca, Gambellini, Taddei, Belardinelli, Guerra, Mazzini and Fausto2015; Pappalardo et al., Reference Pappalardo, D'Urso, Viscuso, Ferrrito, Giunta, Cupani and Vitale2016).
Ovarioles are the units of ovaries where the oocyte grows and developed. The anterior end of the ovariole has a filamentous structure that connects the ovariole to the body wall (Klowden, Reference Klowden2013; Polat, Reference Polat2016). The germarium layer containing germ cells comes after the terminal filament. As germ cells grow and develop, they move toward the anterior end of the ovary. First, the mature eggs are kept at the lateral oviduct until the female finds a suitable place for oviposition. Then, the female pushes the mature egg toward the genital chamber by the contraction of the muscles in the lateral oviduct for oviposition (Lange, Reference Lange2009; Polat, Reference Polat2016). Meanwhile, the female releases the sperm which is from the male insect and stores the sperm in spermatheca after mating, and thus, fertilization is achieved (Lange, Reference Lange1990, Reference Lange2009; Giuffrida & Rosati, Reference Giuffrida and Rosati1993; Viscuso et al., Reference Viscuso, Narcisi and Sottile1999; Vahed, Reference Vahed2003; Sturm, Reference Sturm2005; Brundo et al., Reference Brundo, Longo, Sottile, Trovato, Vitale and Viscuso2011; Polat, Reference Polat2016).
In many insect species like Sitophilus granarius (L.) (Coleoptera, Curculionidae), the females make holes in grains to laying eggs in the holes. Then, the females release secretion to create a kind of plug composed of mucous material which has a role in sticking the eggs to each other and secures them from the grain infestation. This mucous material is secreted by the activity of the female reproductive gland. Likewise, the female reproductive glands have some duties on showing antibacterial properties, carrying sperms into the spermatheca, and producing some materials of the egg chorion (Gaino & Fava, Reference Gaino and Fava1995; Sturm, Reference Sturm2012; Masci et al., Reference Masci, Di Luca, Gambellini, Taddei, Belardinelli, Guerra, Mazzini and Fausto2015; Gnatzy et al., Reference Gnatzy, Volknandt and Dzwoneck2018).
Although many insect species have the main and accessory reproductive organs, their morphological and histological structure, number, and ultrastructure vary between ordos, families, and species. For that reason, these features are very important taxonomically (Nandchahal, Reference Nandchahal1972; Lange, Reference Lange2009; Brundo et al., Reference Brundo, Longo, Sottile, Trovato, Vitale and Viscuso2011; Klowden, Reference Klowden2013; Masci et al., Reference Masci, Di Luca, Gambellini, Taddei, Belardinelli, Guerra, Mazzini and Fausto2015; Polat, Reference Polat2016).
Ordo Orthoptera generally includes phytophagous insects. Some species of Orthoptera can create swarms that can increase their populations very quickly in a short time and migrate over long distances. Therefore, they can cause large losses in agricultural fields and crops. For that reason, ordo Orthoptera forms a group that has an economical importance. In this context, in order to combat invasive species, the structures of these species must be well known (Mead et al., Reference Mead, Khachatourians and Jones1988; Şahin et al., Reference Şahin, Bitmiş and Erman2004; Karaca et al., Reference Karaca, Aslan, Demirözer and Karsavuran2006; Willis et al., Reference Willis, Klingeman, Oppert, Oppert and Jurat-Fuentes2010; Yılmaz et al., Reference Yılmaz, Suludere and Candan2012; Polat, Reference Polat2016).
Poecilimon, a genus belonging to the Tettigoniidae family (Orthoptera), lives on the shrubs in the open areas at the edges of coniferous forests. Genus Poecilimon has 134 species identified in most of the South-Eastern Europe, in Anatolia, and in the Caucasus region. The individuals belonging to this genus have generally green color and short wings that enable them to have very little displacement. The knowledge about the biology and structure of Poecilimon ataturki Ünal, 1999 (Orthoptera, Tettigoniidae) which is an endemic species in Turkey, is very limited in the literature (Sevgili, Reference Sevgili2001; Heller, Reference Heller2004; Heller & Sevgili, Reference Heller and Sevgili2005; Tazegül & Önder, Reference Tazegül and Önder2012; Polat, Reference Polat2016). In line with all this information, the main goal of this study is to reveal the morphological, histological, and cytological structure of the ovaries of P. ataturki.
Material and Methods
The field survey of P. ataturki was done in Bolu province, Hamidiye Village in July 2019, and 10 female P. ataturki individuals were taken to the laboratory after collecting by hand on the shrubs or by sweep net. P. ataturki females were dissected, and the ovaries and eggs were taken out from the body cavity. Then, the ovaries were cleaned, photographed under the stereomicroscope, and prepared for the light microscope (LM), the scanning electron microscope (SEM), and the transmission electron microscope (TEM) examination.
For the LM studies, the ovaries were dehydrated in the ascending series of ethanol and embedded in paraffin blocks. The sections at 6 μm thickness were cut with a microtome and stained with a routine histological staining procedure (Hematoxylin-Eosin, H&E) and Mallory's trichrome staining. They were also stained with Periodic Acid Schiff (PAS) to mark neutral mucosubstances, and Bromophenol Blue (BPB) and Mercury Bromophenol Blue (mBPB) were used to reveal the protein in the oocytes. Then, the slides were examined with an LM (Olympus BX51) and photographed.
For the SEM examinations, the ovaries were first dehydrated and then dried with a critical point dryer (Polaron CPD 7501). The dried specimens of the ovaries were bonded on the SEM stubs and coated with gold with a sputter coater (Polaron SC 502). Afterwards, the ovaries were examined in the SEM (JEOL JSM 6060 LV) and images were recorded.
For the TEM examinations, the ovaries were first taken in 5% glutaraldehyde for pre-fixation, and then, they were post-fixed in 1% OsO4. After rinsing in phosphate buffer, the ovaries were dehydrated in the ascending series of ethanol and blocked in Araldite. Ultra-thin sections at 0.1 μm thickness were cut with an ultramicrotome and stained with uranyl acetate and lead citrate. Finally, the sections were imaged with the TEM (JEOL JEM 1400) and photographed.
Results
The female reproductive system in P. ataturki is composed of a pair of ovaries, a pair of lateral oviducts, a median (common) oviduct, and a receptaculum seminis (spermatheca) (Figs. 1, 2). Ovaries are located dorsolaterally in the body cavity, and a group of the filamentous structure composed of terminal filaments connect the ovaries to the body wall. Lateral oviducts are the canals where the mature eggs are expelled from the ovaries, and both lateral oviducts open to the median oviduct (Fig. 2). The receptaculum seminis which has a duty on storing the sperm until oviposition after copulation is also connected to the median oviduct (Fig. 2).

Fig. 1. The general structure of the female reproductive system in P. ataturki. Ov, ovarium; →, lateral oviduct; ⃰⃰, terminal filament (Stereomicroscope image, scale bar = 2 mm).

Fig. 2. The general structure of the female reproductive system in P. ataturki. Ov, ovarium; →, lateral oviduct; ►, receptaculum seminis (spermatheca); Co, common (median) oviduct (Stereomicroscope image, scale bar = 2 mm).
Each ovary contains approximately 9–12 panoistic type ovarioles (Figs. 1–3). The ovaries are covered by a meshwork called as epithelial sheath composed of the muscle tissue (Fig. 4).

Fig. 3. The general structure of an ovarium. Encircled: ovariol; →, terminal filament; ⃰, epithelial sheath (SEM image, scale bar = 1 mm).

Fig. 4. The ovariol (O) covered with the epithelial sheath (*) (SEM image, scale bar = 100 μm).
The ovarioles are composed of three regions called terminal filament, germarium, and vitellarium. The terminal filaments are filamentous structures at the distal side of the ovariole that come together and connect the ovariole to the body wall (Figs. 1, 3). Germarium is the region that has undifferentiated cells which generate the oocytes and follicular cells (Fig. 5). There is a layer of interfollicular cells at the base of the germarium (Fig. 5). In the LM and TEM micrographs, the cells of the interfollicular layer look elongated and multilayered with elongated nucleus (Figs. 6, 7). Vitellarium comes after germarium and is characterized by a series of ovariole follicles with oocytes in different developmental stages (Fig. 8). Vitellarium consists of three developmental stages including previtellogenesis, vitellogenesis, and choriogenesis. At the end of the choriogenesis, the oocyte completes its development.

Fig. 5. The longitudinal section of the germarium region and interfollicular area of the ovariol. G, germarium; IfC, interfollicular cells; V, vitellarium (LM image, H&E, scale bar = 0.05 mm).

Fig. 6. The longitudinal section of the interfollicular cells with the elongated nucleus (→) (LM image, H&E, scale bar = 0.02 mm).

Fig. 7. The longitudinal section of the interfollicular cells with the elongated nucleus (→) (TEM image, scale bar = 5 μm).

Fig. 8. The previtellogenic stage with the different developmental stages (→) and the choriogenic stage (C) (LM image, Mallory's trichrome staining, scale bar = 0.25 mm).
At the previtellogenesis, the first stage of the vitellarium, the thin follicle cell layer encircles the oocyte (Figs. 8–10). The follicle cells are squamous and single layered, and the nuclei occupy a large part of the follicle cells (Fig. 10). In the previtellogenic stage, the yolk granules and lipid droplets are not yet present in the oocytes.

Fig. 9. The detail of the previtellogenic stage and the choriogenic stage. PV, previtellogenic stage; F, follicle cells of the choriogenic stage; n, nucleus of the oocyte in the previtellogenic stage; Y, yolk granules; →, chorion of the egg; ►, epithelial sheath (LM image, mallory's trichrome staining, scale bar = 0.02 mm).
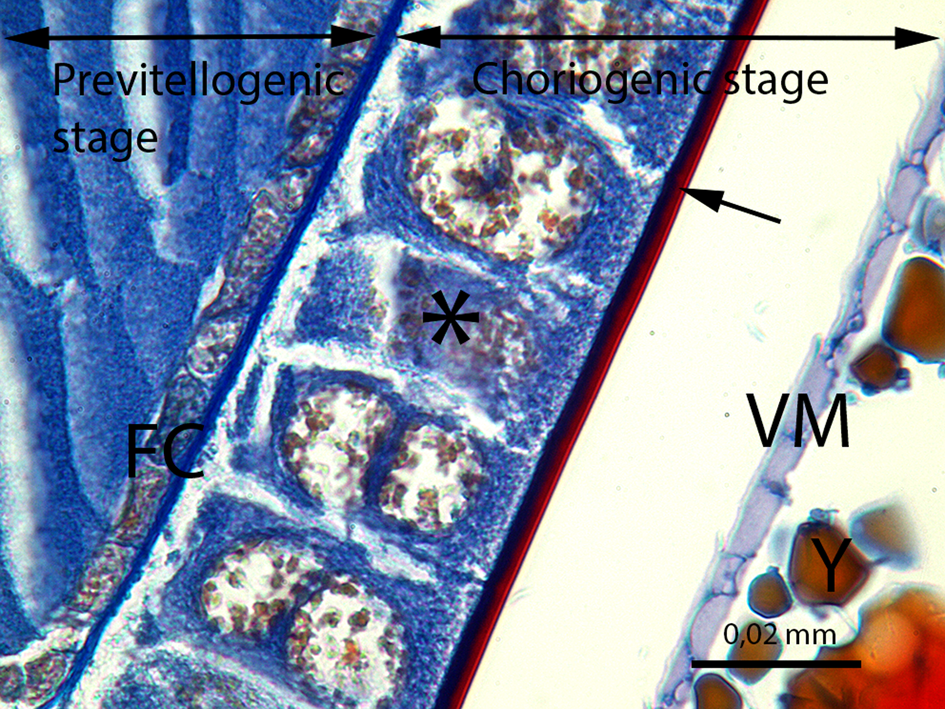
Fig. 10. The developing oocyte in the previtellogenic stage and the oocyte in the choriogenic stage. FC, follicle cells of the previtellogenic stage; ⃰, follicle cells of the choriogenic stage; →, chorion of the egg; VM, vitelline membrane; Y, yolk granules (LM image, Mallory's trichrome staining, scale bar = 0.02 mm).
The second stage of the vitellarium is the vitellogenesis which comes after the previtellogenesis. This stage of development is characterized by the yolk granules produced without the chorion in the oocyte (Figs. 11, 12). Yolk granules begin to be secreted by follicle cells at the beginning of this stage, and the oocyte is full with yolk granules of different sizes and shapes at the end of the vitellogenic stage (Fig. 11). The follicle cells in the vitellogenic stage are single-layered cuboidal-columnar cells (Figs. 13–15). There are some gaps in different wideness between the follicle cells called intercellular spaces (Figs. 13, 14). As intercellular spaces can completely separate the lateral membranes of two follicle cells, they can also be seen in enlarged areas in the lateral membranes in some regions (Figs. 14, 15). In TEM micrographs of the vitellogenic stage, we can observe the large nucleus of the columnar follicle cells. The two-lobed nucleus, which appears to be knuckled in the middle, occupies a large part of the follicle cell. Euchromatin regions are generally predominant in the nucleus (Figs. 15, 16). That the cytoplasm of the follicle cells has numerous granulated endoplasmic reticulum cisterns that can be observed in Figure 17.

Fig. 11. The longitudinal section of the oocyte in the vitellogenic stage. Y, yolk granules; →, follicle cells of vitellogenic stage (SEM image, scale bar = 100 μm).

Fig. 12. The vitellogenic stage in the ovariol without the chorion. FC, follicle cells; Y, yolk granules; N, nucleus (LM image, Mallory's trichrome staining, scale bar = 0.02 mm).

Fig. 13. The vitellogenic stage in the ovariol without the chorion. FC, follicle cells; Y, yolk granules (SEM image, scale bar = 50 μm).

Fig. 14. The vitellogenic stage in the ovariol. FC, follicle cells; Y, yolk granules; ►, intercellular space; →, lateral membrane of the follicle cell (SEM image, scale bar = 10 μm).

Fig. 15. The vitellogenic stage in the ovariol. FC, follicle cells; Y, yolk granules; N, nucleus; ⃰, intercellular space; →, enlarged areas between two lateral membranes (TEM image, scale bar = 10 μm).

Fig. 16. The follicle cell with the two-lobed nucleus (N) in the vitellogenic stage. IS, intercellular space (TEM image, scale bar = 5 μm).

Fig. 17. The cytoplasm of the follicle cell of the choriogenic stage with the granulated endoplasmic reticulum (GER) (TEM image, scale bar = 500 nm).
The last stage of the vitellarium is called as choriogenesis. With the choriogenesis, the vitelline membrane and chorion encircle oocyte are formed (Fig. 10). Thus, the oocyte development is completed and the mature oocyte is transferred from the ovariole into the lateral oviduct. In Figure 18, two layers of the mature oocyte chorion, the endochorion and the exochorion, can be distinguished in the lateral oviduct.

Fig. 18. The longitudinal section of the lateral oviduct (LO). Ex, exochorion; En, endochorion (LM image, H&E, scale bar = 0.02 mm).
According to the results of histochemical studies, it is seen that some of the yolk granules in the ooplasm were stained purple in sections treated with PAS stain. The PAS-positive areas of the yolk in the ooplasm are indicated in Figures 19 and 20. The positive reaction of the PAS stain with carbohydrates of the yolk granules began to be seen in the vitellogenesis stage of oogenesis (Fig. 19). In sections treated with BPB, it is seen that some of the yolk granules are stained blue in the choriogenesis stage of oogenesis (Fig. 21). On the other hand, there was no reaction in the sections treated with mBPB (Fig. 22).

Fig. 19. The PAS staining of the vitellogenic stage of the ovariol. *, PAS-positive carbohydrate yolks; F, follicular epithelium (LM image, PAS staining, scale bar = 0.01 mm).

Fig. 20. The PAS staining of the choriogenic stage of the ovariol. *, PAS-positive carbohydrate yolks; F, follicular epithelium; arrows, chorion (LM image, PAS staining, scale bar = 0.02 mm).

Fig. 21. The BPB staining of the choriogenic stage of the ovariol. *, BPB positive protein yolks; F, follicular epithelium; arrows, chorion (LM image, BPB staining, scale bar = 0.02 mm).

Fig. 22. The negative mBPB staining of the choriogenic stage of the ovariol. There are no mBPB stained blue yolk granules in the ooplasm. *, mBPB negative protein yolks; F, follicular epithelium; arrows, chorion (LM image, mBPB staining, scale bar = 0.02 mm).
Discussion
When ovary morphology in P. ataturki was examined, the first striking and important difference is the number of ovarioles with the exception of color, shape, or size. The number of ovarioles in insects may vary between species in the same order, even in the same family. In Baeacris punctulatus (Thunberg, 1824) (Orthoptera, Acrididae), the ovaries are made up of 10 ovarioles (Michel & Terán, Reference Michel and Terán2005). Gryllodes sigillatus (Walker, 1869) (Orthoptera, Gryllidae) has 120–150 ovarioles in the female reproductive organ (Nandchahal, Reference Nandchahal1972). This number changes between 9 and 12 ovarioles in P. ataturki. Ovariole number is a powerful determinant of fertility rate (Taylor & Whitman, Reference Taylor and Whitman2010; García-Navas et al., Reference García-Navas, Noguerales, Cordero and Ortego2017). The average number of the ovarioles and eggs generally show a strong relationship between insect body size and the number of ovarioles in grasshoppers (Schultner et al., Reference Schultner, Blanchet, Pagès, Lehmann and Lecoq2012). Therefore, female individuals with more ovarioles can produce a great number of eggs at each fertilization. However, the reproductive capacity of a female individual can be influenced by a number of other factors, such as the environmental factors, feeding style, or genetic factors.
In this paper, the ovarian histology was also mentioned in addition to its morphology. The Ovarian histology in P. ataturki was found similar to the ovarian histology of other studied species belonging to the order Orthoptera. No significant difference was found in the cellular structure. The subject which is wanted to be highlighted in this study is the content of the yolk granules in the oocyte cytoplasm (ooplasm).
The proteins, lipids, and carbohydrates deposited in the oocyte during the maturation of the egg (Shende & Masram, Reference Shende and Masram2020). These storing occur in storage granules founded in the ooplasm of the oocytes, and the ooplasmic granules start to show up in the oocytes in the beginning stages of the development (Anholeto et al., Reference Anholeto, de Oliveira, Rodrigues, Yamane, Castro and Camargo-Mathias2018; Shende & Masram, Reference Shende and Masram2020). These stored granules, called as the yolk granules, give on to quick growth of the oocytes (Shende & Masram, Reference Shende and Masram2020).
It is known that the carbohydrates are synthesized in the ovary as glycogen and used during the growing of the oocyte (Hilker & Meiners, Reference Hilker and Meiners2008; Sanjayan, Reference Sanjayan and Anantanarayanan2018). With the PAS staining was used which determines mucosubstances, the yolk granules in the ooplasm gave a PAS-positive reaction, and consequently, it was deduced that the content of the yolk granules is carbohydrates. Although carbohydrate yolks were observed in large quantities only during the later stage of vitellogenesis in ooplasm, the mechanism of its formation starts during the earliest stages. This shows that more carbohydrates are needed at the last stage of egg formation.
In the histochemical observations to demonstrate the yolk protein, two different BPB stainings were used as mBPB and BPB. The mercury ions react with acid groups in proteins, causing the yolk granules to turn blue in mBPB. However, we concluded that the proteins were not acidic since the yolk granules were not stained with mBPB in P. ataturki. Nonetheless, BPB staining was supposed to turn blue by reacting with the basic groups in proteins. As a result of the negative control after staining, the yolk proteins in P. ataturki were stained blue and it was revealed that these proteins were basic. The appearance of BPB positive staining from the germinal vesicle not folicle cells indicate a source of protein, synthesized by the germinal vesicle in ooplasm.
In the TEM observations, the fact that we encountered the well-developed granulated endoplasmic reticulum in the cytoplasm of the follicle cells supports the conclusion that the formation of the yolk granules is initiated by the granulated endoplasmic reticulum (Roth & Porter, Reference Roth and Porter1964; Dettlaff & Vassetzky, Reference Dettlaff and Vassetzky2012).
Conclusion
In the present study, we revealed the morphology, histology of P. ataturki's ovary, and the histochemistry of yolk granules in the oocyte cytoplasm. We hope that our results will contribute to further studies.
Acknowledgments
We express our thanks to Prof. Dr. Mustafa ÜNAL (Bolu Abant Izzet Baysal University, Faculty of Arts and Sciences, Biology Department) for helping with the species diagnosis and to Gazi University Academic Writing and Research Center for their help and support in the proofreading of the current study.