No CrossRef data available.
Article contents
Transmission Electron Microscopy as a Tool to Study the Toxicological Effects of Thiamethoxam in Workers of Atta sexdens (Myrmicinae, Attini)
Published online by Cambridge University Press: 07 December 2020
Abstract
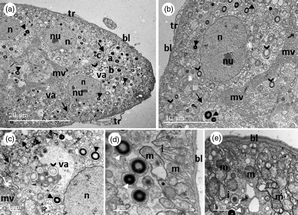
Thiamethoxam is a neonicotinoid that has been used to control insect pests. The literature reports a few behavioral studies evaluating the toxic effect of thiamethoxam in ants; however, there are scarce studies at the cellular level. The present research evaluated the effects of thiamethoxam in labial (LG) and mandibular glands (MG), fat bodies (FB), and Malpighian tubules (MT) of workers of Atta sexdens, using transmission electron microscopy. The duct and secretory cells of LG were profoundly affected, then the production of saliva can be compromised, as well as its quality and subsequent use. In MG, reservoir and canaliculi cells presented slight alterations; however, MG secretory cells presented vacuoles containing lamellar structures, increased lipid production, and a large amount of mitochondria, which may lead to organ's malfunctioning. The FB cell alterations do not seem enough to cause significant changes that lead to cell death. Prominent changes in MT, such as loss of the electron-dense concentric ring, increased smooth endoplasmic reticulum, loss of basal infolds, vacuoles containing mineralized granules, and lamellar structures associated with mitochondria, suggest that their excretory function is compromised. In conclusion, thiamethoxam acts not only in the nervous system but also contributes to systemic toxicity on the target organism.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of the Microscopy Society of America





