Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Taylor, H. F. W.
1973.
Crystal structures of some double hydroxide
minerals.
Mineralogical Magazine,
Vol. 39,
Issue. 304,
p.
377.
Pies, W.
and
Weiss, A.
1974.
References for III/7.
Vol. 7g,
Issue. ,
p.
425.
Burns, R. G.
Burns, V. M.
Usdowski, H. E.
and
Mueller, R. F.
1974.
Handbook of Geochemistry.
Vol. 2 / 4,
Issue. ,
p.
125.
Pies, W.
and
Weiss, A.
1977.
Key Elements: d9-, d10-, d1_d3-, f-Elements.
Vol. 7e,
Issue. ,
p.
425.
Meyer, H. J.
Pies, W.
and
Weiss, A.
1979.
Key Element: C.
Vol. 7c3,
Issue. ,
p.
174.
Livingstone, A.
and
Bish, D. L.
1982.
On the new mineral theophrastite, a nickel hydroxide, from Unst, Shetland, Scotland.
Mineralogical Magazine,
Vol. 46,
Issue. 338,
p.
1.
Mattson, Stephanie M.
and
Rossman, George R.
1984.
Ferric iron in tourmaline.
Physics and Chemistry of Minerals,
Vol. 11,
Issue. 5,
p.
225.
Hernandez-Moreno, Maria J.
Ulibarri, María A.
Rendon, J. L.
and
Serna, Carlos J.
1985.
IR characteristics of hydrotalcite-like compounds.
Physics and Chemistry of Minerals,
Vol. 12,
Issue. 1,
p.
34.
Drits, V. A.
Sokolova, T. N.
Sokolova, G. V.
and
Cherkashin, V. I.
1987.
New Members of the Hydrotalcite-Manasseite Group.
Clays and Clay Minerals,
Vol. 35,
Issue. 6,
p.
401.
Schaper, H.
Berg-Slot, J.J.
and
Stork, W.H.J.
1989.
Stabilized magnesia: A novel catalyst (support) material.
Applied Catalysis,
Vol. 54,
Issue. 1,
p.
79.
Grey, Ian E.
and
Ragozzini, Roland
1991.
Formation and characterization of new magnesium aluminum hydroxycarbonates.
Journal of Solid State Chemistry,
Vol. 94,
Issue. 2,
p.
244.
Hofmeister, W.
and
Platen, H. Von
1992.
Crystal Chemistry and Atomic Order in Brucite-related Double-layer Structures.
Crystallography Reviews,
Vol. 3,
Issue. 1,
p.
3.
de Roy, André
Forano, Claude
Malki, Khalid El
and
Besse, Jean-Pierre
1992.
Expanded Clays and Other Microporous Solids.
p.
108.
de Roy, André
Forano, Claude
Malki, Khalid El
and
Besse, Jean-Pierre
1992.
Expanded Clays and Other Microporous Solids.
p.
108.
El Malki, K.
de Roy, A.
and
Besse, J.P.
1993.
Evolution related to hygrometry of two lamellar double hydroxide pillared structures [CuCrS04] and [ZnAlS04].
Nanostructured Materials,
Vol. 2,
Issue. 2,
p.
169.
Evans, David G.
and
Slade, Robert C. T.
2005.
Layered Double Hydroxides.
Vol. 119,
Issue. ,
p.
1.
Villars, P.
Cenzual, K.
Daams, J.
Gladyshevskii, R.
Shcherban, O.
Dubenskyy, V.
Melnichenko-Koblyuk, N.
Pavlyuk, O.
Savysyuk, I.
Stoyko, S.
and
Sysa, L.
2007.
Structure Types. Part 5: Space Groups (173) P63 - (166) R-3m.
Vol. 43A5,
Issue. ,
p.
667.
Frost, Ray L.
Bahfenne, Silmarilly
and
Graham, Jessica
2008.
Infrared and infrared emission spectroscopic study of selected magnesium carbonate minerals containing ferric iron—Implications for the geosequestration of greenhouse gases.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 71,
Issue. 4,
p.
1610.
Britvin, Sergey N.
2008.
Minerals as Advanced Materials I.
p.
123.
Frost, Ray L.
Bahfenne, Silmarilly
Graham, Jessica
and
Martens, Wayde N.
2008.
Thermal stability of artinite, dypingite and brugnatellite—Implications for the geosequestration of green house gases.
Thermochimica Acta,
Vol. 475,
Issue. 1-2,
p.
39.
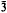 m, aH = 3·12, cH = 37·4 Å, Z = ½, and (0001) cleavage. The structure is of a layer type, and is based on a structural element about 12·5 Å thick in the c-direction and consisting of two brucite-like layers and one disordered layer containing carbonate ions and water molecules and resembling those in sjögrenite and pyroaurite. The unit cell comprises three of these structural elements stacked together in the c-direction. The Mg2+ and Fe3+ ions are randomly distributed among all the octahedral sites of the brucite-like layers. The structure closely resembles those of sjögrenite and pyroaurite, but has two brucite-like layers between each CO32−−H2O layer where these have one. There is a tendency to random interstratification, and the crystals appear to contain intergrown regions of brucite and of sjögrenite or pyroaurite. Coalingite-K probably has a similar structure, but with three brucite-like layers between each
m, aH = 3·12, cH = 37·4 Å, Z = ½, and (0001) cleavage. The structure is of a layer type, and is based on a structural element about 12·5 Å thick in the c-direction and consisting of two brucite-like layers and one disordered layer containing carbonate ions and water molecules and resembling those in sjögrenite and pyroaurite. The unit cell comprises three of these structural elements stacked together in the c-direction. The Mg2+ and Fe3+ ions are randomly distributed among all the octahedral sites of the brucite-like layers. The structure closely resembles those of sjögrenite and pyroaurite, but has two brucite-like layers between each CO32−−H2O layer where these have one. There is a tendency to random interstratification, and the crystals appear to contain intergrown regions of brucite and of sjögrenite or pyroaurite. Coalingite-K probably has a similar structure, but with three brucite-like layers between each 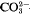 -H2O layer; its idealized formula is probably Mg16Fe2(OH)36(CO3).2H2O.
-H2O layer; its idealized formula is probably Mg16Fe2(OH)36(CO3).2H2O.



