Article contents
D'ansite-(Mn), Na21Mn2+(SO4)10Cl3 and d'ansite-(Fe), Na21Fe2+(SO4)10Cl3, two new minerals from volcanic fumaroles
Published online by Cambridge University Press: 05 July 2018
Abstract
The new minerals d'ansite-(Mn), Na21Mn2+(SO4)10Cl3, and d'ansite-(Fe), Na21Fe2+(SO4)10Cl3, occur as encrustations in fumaroles at Vesuvius, Naples, Italy and La Fossa crater, Vulcano, Aeolian Islands, Italy, respectively. Both minerals are cubic and crystallize in space group I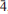 3d. D'ansite-(Mn) forms colourless translucent tristetrahedral crystals up to 0.2 mm on edge; d'ansite-(Fe) forms aggregates of colourless to white complex isometric crystals of about the same size. Chemical analyses obtained by energy-dispersive spectrometry on an electron microprobe gave the following mean compositions: d'ansite-(Mn), Na2O 39.37, MnO 3.46, MgO 0.13, SO3 49.99, Cl 6.36, O=Cl–1.44, total 97.87 wt.%, corresponding to an empirical formula, on the basis of 43 anions, of Na20.61 (Mn2+0.79Mg0.05)Σ0.84S10.13O40.09Cl2.91; and d'ansite-(Fe), Na2O 39.12, FeO 4.18, MgO 0.12, SO3 49.91, Cl 6.81, O=Cl –1.54, total 98.60 wt.%, corresponding to an empirical formula of Na20.42(Fe2+0.94Mg0.05)Σ0.99S10.08O39.89Cl3.11. The six strongest reflections in the X-ray powder diffraction pattern of d'ansite-(Fe) [listed as dobs(Å) (I) (hkl)] are as follows: 2.807(100)(044), 2.570(37)(235), 1.714(29)(129), 3.384(27)(233), 3.113(26)(134), 2.108(15)(237). The unit-cell parameters obtained from single-crystal data are 15.9291(9) and 15.882(3) Å for d'ansite-(Mn) and d'ansite-(Fe), respectively. The structure of both minerals was refined, using single-crystal diffraction data, to final R parameters of 0.0309 and 0.0336 on reflections with I > 2σ(I). The structure contains three independent Na sites, one of which is partially occupied by Mn2+ or Fe2+, two independent sulfate anions and one chlorine site.
3d. D'ansite-(Mn) forms colourless translucent tristetrahedral crystals up to 0.2 mm on edge; d'ansite-(Fe) forms aggregates of colourless to white complex isometric crystals of about the same size. Chemical analyses obtained by energy-dispersive spectrometry on an electron microprobe gave the following mean compositions: d'ansite-(Mn), Na2O 39.37, MnO 3.46, MgO 0.13, SO3 49.99, Cl 6.36, O=Cl–1.44, total 97.87 wt.%, corresponding to an empirical formula, on the basis of 43 anions, of Na20.61 (Mn2+0.79Mg0.05)Σ0.84S10.13O40.09Cl2.91; and d'ansite-(Fe), Na2O 39.12, FeO 4.18, MgO 0.12, SO3 49.91, Cl 6.81, O=Cl –1.54, total 98.60 wt.%, corresponding to an empirical formula of Na20.42(Fe2+0.94Mg0.05)Σ0.99S10.08O39.89Cl3.11. The six strongest reflections in the X-ray powder diffraction pattern of d'ansite-(Fe) [listed as dobs(Å) (I) (hkl)] are as follows: 2.807(100)(044), 2.570(37)(235), 1.714(29)(129), 3.384(27)(233), 3.113(26)(134), 2.108(15)(237). The unit-cell parameters obtained from single-crystal data are 15.9291(9) and 15.882(3) Å for d'ansite-(Mn) and d'ansite-(Fe), respectively. The structure of both minerals was refined, using single-crystal diffraction data, to final R parameters of 0.0309 and 0.0336 on reflections with I > 2σ(I). The structure contains three independent Na sites, one of which is partially occupied by Mn2+ or Fe2+, two independent sulfate anions and one chlorine site.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2016
References
- 5
- Cited by




