Article contents
Mechanism of Ion Exclusion by Sub-2nm Carbon Nanotube Membranes
Published online by Cambridge University Press: 01 February 2011
Abstract
Carbon nanotubes offer an outstanding platform for studying molecular transport at nanoscale, and have become promising materials for nanofluidics and membrane technology due to their unique combination of physical, chemical, mechanical, and electronic properties. In particular, both simulations and experiments have proved that fluid flow through carbon nanotubes of nanometer size diameter is exceptionally fast compared to what continuum hydrodynamic theories would predict when applied on this length scale, and also, compared to conventional membranes with pores of similar size, such as zeolites.
For a variety of applications such as separation technology, molecular sensing, drug delivery, and biomimetics, selectivity is required together with fast flow. In particular, for water desalination, coupling the enhancement of the water flux with selective ion transport could drastically reduce the cost of brackish and seawater desalting. In this work, we study the ion selectivity of membranes made of aligned double-walled carbon nanotubes with sub-2 nm diameter. Negatively charged groups are introduced at the opening of the carbon nanotubes by oxygen plasma treatment.
Reverse osmosis experiments coupled with capillary electrophoresis analysis of permeate and feed show significant anion and cation rejection. Ion exclusion declines by increasing ionic strength (concentration) of the feed and by lowering solution pH; also, the highest rejection is observed for the 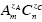 salts (A=anion, C=cation, z= valence) with the greatest zA/zC ratio. Our results strongly support a Donnan-type rejection mechanism, dominated by electrostatic interactions between fixed membrane charges and mobile ions, while steric and hydrodynamic effects appear to be less important. Comparison with commercial nanofiltration membranes for water softening reveals that our carbon nanotube membranes provides far superior water fluxes for similar ion rejection capabilities.
salts (A=anion, C=cation, z= valence) with the greatest zA/zC ratio. Our results strongly support a Donnan-type rejection mechanism, dominated by electrostatic interactions between fixed membrane charges and mobile ions, while steric and hydrodynamic effects appear to be less important. Comparison with commercial nanofiltration membranes for water softening reveals that our carbon nanotube membranes provides far superior water fluxes for similar ion rejection capabilities.
Keywords
- Type
- Research Article
- Information
- MRS Online Proceedings Library (OPL) , Volume 1106: Symposium PP – The Business of Nanotechnology , 2008 , 1106-PP03-03
- Copyright
- Copyright © Materials Research Society 2008
References
- 11
- Cited by




