Article contents
Synchrotron X-ray powder diffraction pattern of the M8 murataite polytype
Published online by Cambridge University Press: 21 June 2017
Abstract
The authors present here the superior result of synchrotron X-ray diffraction indexing of the 8 × 8 × 8 fluorite supercell, murataite, M8 (formula Ca66.37Mn29.33Ti186.76Zr83.35Al46.30Fe74.09O817, a = 39.277(3) Å, Z = 4). The authors present evidence that Fd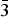 $\bar 3$m (227) is the most likely space group.
$\bar 3$m (227) is the most likely space group.
Keywords
- Type
- Rapid Communication
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2017
References
- 2
- Cited by





