Introduction
Severe hypoglycemia, characterized by impaired consciousness, affects up to 35%-42% of those with Type I diabetes and 16.5% of those with Type II diabetes.Reference Kalra, Mukherjee and Ramachandran1,Reference Villani, de Courten and Zoungas2 Hypoglycemic-induced impaired consciousness can lead to neurologic sequalae or death if not treated promptly.Reference Inkster and Frier3,4 Emergency Medical Services (EMS) are often called to provide care for the patient with severe hypoglycemia. These types of responses account for one percent to four percent of call volumes.Reference Villani, de Courten and Zoungas2
Paramedics treat severe hypoglycemia with intravenous (IV) dextrose.Reference Hern, Kiefer, Louie, Barger and Alter5 Current EMS standard of care is to administer 25g of 50% dextrose (D50).Reference Rostykus, Kennel and Adair6 This dose of D50 is meant to ensure expedited delivery of much needed glucose to the brain in an amount that ensures a return to consciousness. The current literature suggests other options may be as effective with fewer adverse effects. A systematic review conducted in 2008 concluded that 10% dextrose (D10) may be as effective as higher concentration solutions.Reference Nehme and Cudini7
Ten percent dextrose could be considered an alternative to D50 treatment.Reference Nehme and Cudini7 Both D50 and D10 are delivered intravenously, resulting in a rapid response to medication administration. A concern regarding IV delivery of D50 is the risk of extravasation resulting in tissue necrosis. Due to D50 being administered in a hypertonic state, this can result in a high risk of damage to the surrounding tissue when compared to D10, which has more isotonic properties.Reference Hern, Kiefer, Louie, Barger and Alter5 The 25g dose of D50 provides five-times the normal adult level of glucose.Reference Kleinman, Chameides and Schexnayder8 This high concentration delivery can often lead to post-treatment hyperglycemia and unnecessary physiological challenges for the patient in the time following the event.Reference Rostykus, Kennel and Adair6 Furthermore, D50 is in frequent short supply and costly, at approximately US$7.00 per ampule compared to US$2.50 per 25g bag, making D10 a sensible consideration.Reference Rostykus, Kennel and Adair6,9 The 2018 United Kingdom Joint Royal Colleges Ambulance Liaison Committee (London, UK) Guidelines update and the Ambulance Victoria Guideline in Victoria, Australia include D10 as the preferred treatment choice for some of these reasons.10,11
It is hypothesized that alternative treatment modalities such as D10 or titrating the D50 to a complete return of consciousness may be optimal and may avoid some of the complications that arise from larger, more concentrated boluses.
Methods
Study Design
This is a systematic review and meta-analysis of the evidence on the effectiveness and harms of D50 compared to D10 or titrated dextrose for the EMS management of hypoglycemia. The Prehospital Evidence-Based Practice (PEP) program is a knowledge translation program, maintained by the Dalhousie Department of Emergency Medicine, Division of EMS (Halifax, Nova Scotia, Canada). The Division of EMS supported this systematic review. The Cochrane Handbook for Systematic Reviews and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist guided the review and reporting, respectively.Reference Moher, Shamseer and Clarke12 The protocol was registered on PROSPERO, registration number: CRD42019138770 on August 16, 2019. This review did not include collection of patient data and thus did not require ethics approval.
Search Strategy
Databases searched included PubMed (National Center for Biotechnology Information, National Institutes of Health; Bethesda, Maryland USA), Embase (Elsevier; Amsterdam, Netherlands), CINAHL (EBSCO Information Services; Ipswich, Massachusetts USA), and The Cochrane Central Register of Controlled Trials (The Cochrane Collaboration; London, United Kingdom). A search strategy was created with the aid of a health sciences librarian using the medical sub-headings of “Dextrose,” “D50,” “D10,” and “Hypoglycemia.” A full search strategy is available in Appendix A (available online only). Databases were searched from inception to September 15, 2020. A targeted grey literature and on-going trial search were also conducted by author JS from June 17, 2019 through June 18, 2019. This search was conducted using the databases: Cochrane Library (The Cochrane Collaboration; London, United Kingdom); Australian New Zealand Clinical Trials Registry (ANZCTR; Camperdown, New South Wales, Australia); ClinicalTrails.gov (US National Library of Medicine; Bethesda, Maryland USA); Health Canada’s clinical trials database (Health Canada; Ottawa, Ontario, Canada); European Union clinical trials register (EU; Brussels, Belgium); and the PEP. Reference lists from included studies were hand searched.
Study Selection
Studies were considered eligible for inclusion by a priori criteria if the patient population studied received IV dextrose for the treatment of suspected hypoglycemia. Hypoglycemia treatments of interest included the standard 25g ampule diluted to D50 solution, D10, and any other variations of IV dextrose concentrations or titrations. Only studies on adult patients (>18 years of age) with hypoglycemia of any etiology were to inform a range of situations in which dextrose treatment would be considered. The original study setting criteria were limited to only patients treated in the prehospital environment. However, the criteria were later expanded upon to include treatment within the emergency department to be more inclusive.
At least one of the a priori outcomes of interest must have been reported by the included studies. These outcomes were based on previous work on treatment of hypoglycemia completed by the International Liaison Committee on Resuscitation (ILCOR; world-wide organizations).13 The primary outcomes of interest were: resolution of symptoms, time to resolution of symptoms, and blood glucose level (BGL) post-treatment. Level of consciousness may be measured by a formal scale (eg, Glasgow Coma Scale [GCS]) or by clinician impression. Secondary outcomes of interest were: need for subsequent doses, adverse events, resolution of hypoglycemia, and time to resolution of hypoglycemia. Adverse events considered included treatment failure to return consciousness, reverting to standard of care after a failure of initial treatment, or tissue damage. Subsequent doses may be considered as part of the same acute event while attempting to obtain symptom resolution.
Randomized controlled trials (RCTs), cohort studies, observational studies, case series, and case reports were eligible for inclusion. Case studies were specifically considered to capture any report of low incidence adverse events. From the preliminary investigation of this topic, there was an anticipated paucity of prospective and randomized studies, and given the nature of this treatment, it was decided that observational studies would be included. Incomplete or unpublished studies, abstracts, trial protocols, studies on animals, and studies conducted in a laboratory setting were excluded. All languages were included where an English title was available during screening.
All potentially eligible studies were independently screened by two appraisers at the title and abstract level by MH and JS using Covidence software (Melbourne, Victoria).14 Disagreements were resolved by third author adjudication (JAG). Full-text review for inclusion was conducted by MH and JS with third party review by JAG.
Data Extraction and Assessment
Authors MH and JS each independently extracted data from all studies. Where required, data were estimated from the graphs provided. In cases of missing data, corresponding authors were contacted or attempts to contact were made. All data collection was audited by JAG and disagreements were resolved by consensus.
Variables
Data related to study design, dates, setting/location, and sample demographics including but not limited to sample size and setting were extracted. Patient conditions (eg, initial level of consciousness, initial BGL, and history of diabetes) were collected. Intervention and comparison characteristics (eg, drug, concentration, dose, and route) were collected. Results from each reported outcome in the a priori list were collected for analysis.
Risk of Bias
Risk of bias for each study was assessed independently by MH and JS using the Joanna Briggs Institute (JBI; Adelaide, Australia) Critical Appraisal checklists appropriate for each study design.15 The final score was discussed between authors (MH, JS, and JAG), and upon agreement, the final risk of bias score was recorded being very high risk of bias, high risk of bias, medium risk of bias, or low risk of bias. Appendix B (available online only) shows full risk of bias within each study.
Data Synthesis
Study results were compared, where appropriate. Descriptive statistics were reported including mean differences and 95% confidence intervals (CI). Results were pooled across outcomes for both D10 and D50. Graphical representations were used, where appropriate. Formal meta-analysis was not possible due to inconsistent comparison groups rendering statistical synthesis inappropriate.
Results
Study Selection
Six-hundred-eighty studies were identified for inclusion. Full-text review was performed on 51, of which 11 were included for analysis (Figure 1). One non-English language study was translated from German to English by a volunteer. Five identified studies were ultimately excluded because corresponding authors were unable to provide the missing data required to include each study at the full-text phase. Reviewer kappa was 0.55619 for title and abstract screening between authors (MH and JS). For full-text screening, the senior author (JAG) double checked all inclusion decisions and the kappa at this stage was 1.00 between (JS and JAG) and 0.74194 between (MH and JAG).
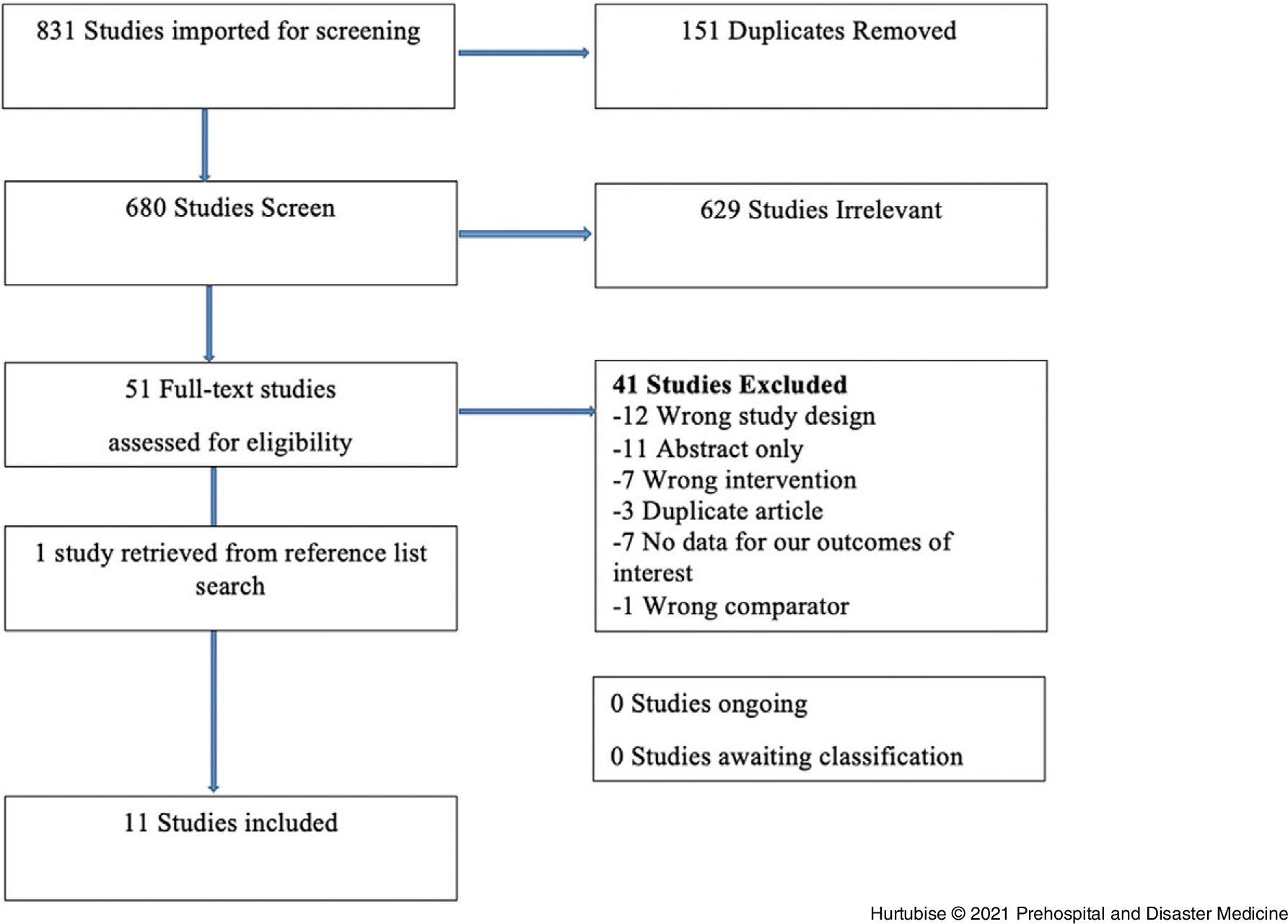
Figure 1. PRISMA Flow Diagram of Included and Excluded Studies.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study Characteristics
The review included 11 studies: three RCTsReference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18 and eight observational studiesReference Hern, Kiefer, Louie, Barger and Alter5,Reference Chinn and Colella19–Reference Weant, Deloney, Elsey, Combs and French25 with a total of 1,462 patients. Of the 11 studies, eight were conducted in the prehospital settingReference Moore and Woollard17,Reference Chinn and Colella19,Reference Hoffman, Schriger, Votey and Luo20,Reference Howell and Guly22–Reference Weant, Deloney, Elsey, Combs and French25 and originated from six countries (Denmark, United States, Wales, England, Germany, and Scotland; Table 1).
Table 1. Characteristics of Included Studies
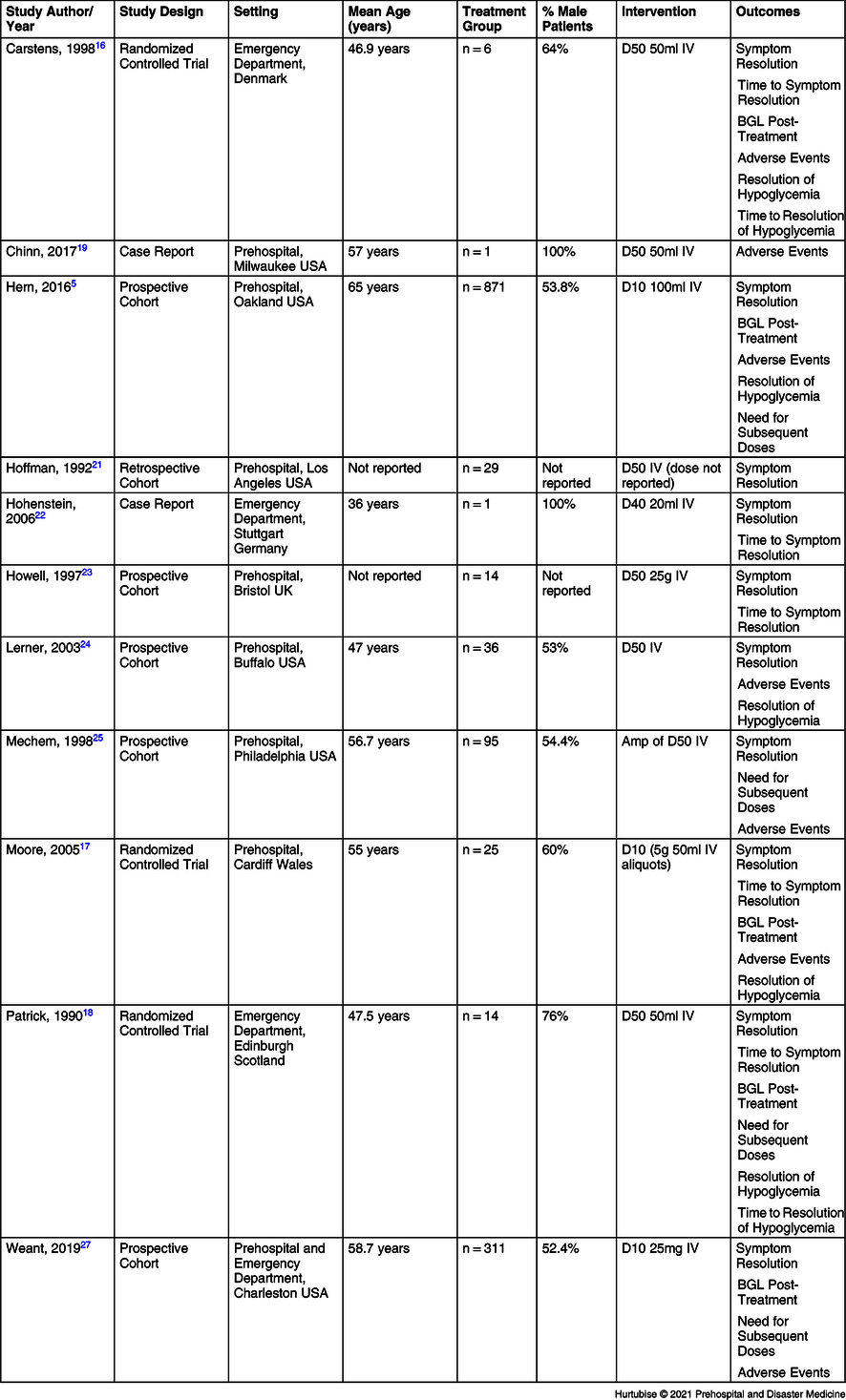
Abbreviations: IV, intravenous; BGL, blood glucose level; D10, 10% dextrose; D40, 40% dextrose; D50, 50% dextrose.
Three studies investigated the effects of D10,Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Moore and Woollard17,Reference Weant, Deloney, Elsey, Combs and French25 nine studies investigated D50,Reference Carstens and Sprehn16–Reference Hoffman, Schriger, Votey and Luo20,Reference Howell and Guly22–Reference Weant, Deloney, Elsey, Combs and French25 and one study investigated a 40% dextrose (D40) solution.Reference Hohenstein21 Two studies compared D10 directly to D50:Reference Moore and Woollard17,Reference Weant, Deloney, Elsey, Combs and French25 Moore was an RCT where both interventions were used, and the dose could be titrated to the clinician’s discretion in response to drug effect;Reference Moore and Woollard17 Weant was an observational before-and-after study where a new protocol supported clinician discretion on dextrose concentration selection.Reference Weant, Deloney, Elsey, Combs and French25 The remaining studies did not have a comparatorReference Hern, Kiefer, Louie, Barger and Alter5,Reference Hoffman, Schriger, Votey and Luo20,Reference Hohenstein21,Reference Lerner23,Reference Mechem24 or used another standard hypoglycemia treatment as the comparator (one milligram of intramuscular glucagon; Table 1).Reference Carstens and Sprehn16,Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Howell and Guly22
Hypoglycemia was defined as point-of-care capillary measurement of less than 3.0 millimole/liter (mmol/L) at the lowest thresholdReference Carstens and Sprehn16 and up to 4.4mmol/L at the higher threshold,Reference Lerner23,Reference Mechem24 or by the paramedic’s “empiric impression” in one study (Hoffman). All studies that recorded history of diabetes reported 100% diabetes etiology, except Moore, which reported 84% (43/51) of patients had insulin dependent diabetes.Reference Moore and Woollard17 One study reported tissue extravasation.Reference Chinn and Colella19
Data for each outcome of interest were extracted. Ten studies investigated symptom resolution;Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Hoffman, Schriger, Votey and Luo20–Reference Weant, Deloney, Elsey, Combs and French25 six investigated time to symptom resolution;Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Hohenstein21,Reference Howell and Guly22,Reference Weant, Deloney, Elsey, Combs and French25 glycemic profile post-treatment was investigated by five studies;Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Weant, Deloney, Elsey, Combs and French25 need for subsequent doses by four studies;Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Mechem24,Reference Weant, Deloney, Elsey, Combs and French25 adverse events by seven studies;Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Carstens and Sprehn16,Reference Moore and Woollard17,Reference Chinn and Colella19,Reference Lerner23–Reference Weant, Deloney, Elsey, Combs and French25 resolution of hypoglycemia by five studies;Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Lerner23 and time to resolution of hypoglycemia was reported in two studiesReference Carstens and Sprehn16,Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18 (Table 2).
Table 2. Studies Investigating Each Outcome of Interest
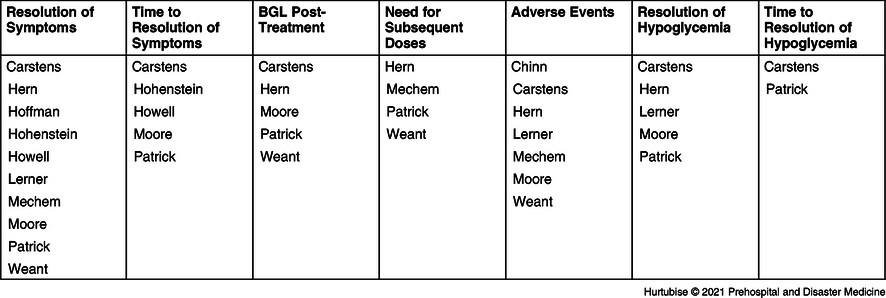
Abbreviation: BGL, blood glucose level.
Risk of Bias within Studies
Using the JBI risk of bias assessment tools, two studies were identified as high risk: one RCT and one retrospective case series.Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Hoffman, Schriger, Votey and Luo20 The Patrick study was deemed high risk due to lack of clarity in reporting of its methods, resulting in concerns regarding valid randomization strategy, blinding and appropriate design execution.Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18 The second high risk of bias study to be assessed was the Hoffman retrospective case series. This study was deemed high risk due to its limited reporting of patient results, patient test group similarities, and patient condition prior to treatment.Reference Hoffman, Schriger, Votey and Luo20 The remaining studies were all assessed as low risk of bias, included the RCT comparing D10 directly to D50 (Appendix A; available online only).Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Carstens and Sprehn16,Reference Moore and Woollard17,Reference Chinn and Colella19,Reference Hohenstein21–Reference Weant, Deloney, Elsey, Combs and French25
Results of Individual Studies
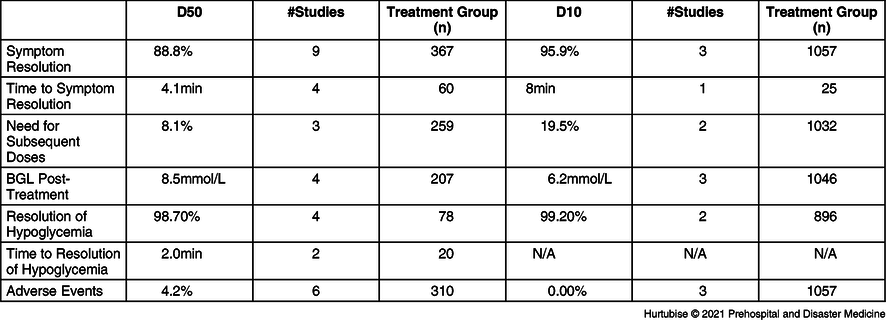
Resolution of Symptoms—All studies reported on symptom resolution after dextrose treatment. Evidence from nine pooled studies (three RCTs and six observational studies), enrolling 367 hypoglycemic patients treated with IV D50, reported symptom resolution in 88.8% (95% CI, 88.7%-89.1%) of patients (Figure 2).Reference Carstens and Sprehn16–Reference Hoffman, Schriger, Votey and Luo20,Reference Howell and Guly22–Reference Weant, Deloney, Elsey, Combs and French25 Evidence from three studies (one RCT and two observational), enrolling 1,057 patients treated with IV D10, reported 95.9% resolution of symptoms (95% CI, 95.8%-95.9%).Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Moore and Woollard17,Reference Weant, Deloney, Elsey, Combs and French25 The RCT comparing D10 to D50 reported a non-statistically significant difference in symptom resolution of four percent favoring D50 (-12 to +22%; P = .360).Reference Moore and Woollard17 Symptom resolution was 96% (25/26 patients) in the D50 arm and 92% (23/25) the D10 arm.Reference Moore and Woollard17 When the results for symptom resolution were pooled across studies, both D10 and D50 had high resolution success with a non-clinically significant difference of 7.1% favoring D10.Reference Carstens and Sprehn16–Reference Hoffman, Schriger, Votey and Luo20,Reference Howell and Guly22–Reference Weant, Deloney, Elsey, Combs and French25 One single case reported a patient received a D40 infusion and recovered to normal level of consciousness.Reference Hohenstein21
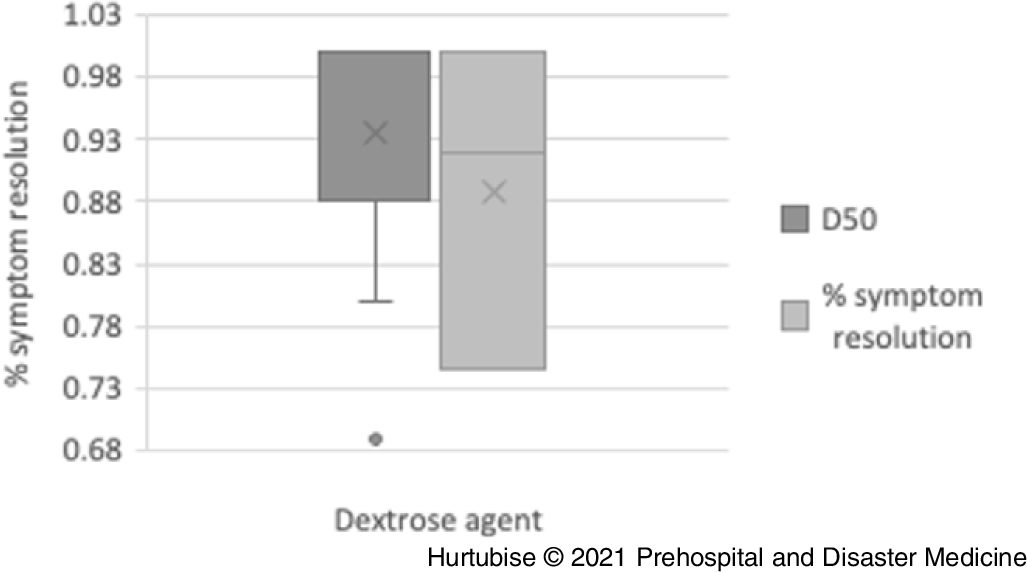
Figure 2. Symptom Resolution.
Abbreviation: D50, 50% dextrose.
Time to Symptom Resolution—Evidence from four studies (three RCTs and one observational), enrolling 60 patients treated with D50, had a mean symptom resolution time of 4.1 minutes (95% CI, 3.46-4.78 minutes; Figure 3).Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Hohenstein21 Evidence from one RCT, enrolling 51 patients treated with dextrose, reported a median time to resolution of 8.0 minutes in the D10 arm (n = 25) and 8.0 minutes in the D50 arm (n = 26; P = .733).Reference Moore and Woollard17 The mean difference between these treatments may be clinically meaningful at 3.9 minutes. One single case reported a patient received a D40 infusion had symptom resolution within two minutes.Reference Hohenstein21
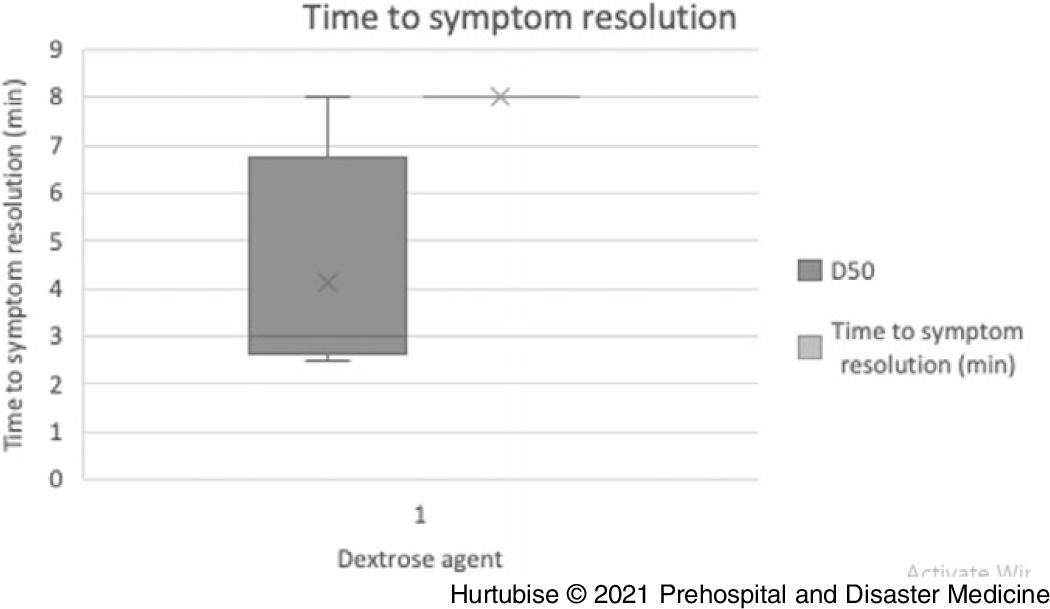
Figure 3. Time to Symptom Resolution.
Abbreviation: D50, 50% dextrose.
Blood Glucose Level Post-Treatment—Evidence from four pooled studies (three RCT and one observational), enrolling 196 total patients treated with D50, resulted in a mean post-treatment glycemic profile of 8.45mmol/L (95% CI, 8.4-8.5mmol/L; Figure 4).Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Weant, Deloney, Elsey, Combs and French25 Evidence from three pooled studies (one RCT and two observational), enrolling 1,057 patients treated with D10, had a mean post-treatment glycemic profile of 6.2mmol/L (95% CI, 6.24-6.16mmol/L).Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Moore and Woollard17,Reference Weant, Deloney, Elsey, Combs and French25 A statistically significant difference of 3.2 minutes (P = .003) was reported by the RCT comparing D50 directly to D10.Reference Moore and Woollard17 This RCT reported a median post-treatment BGL of 9.40mmol/L in the D50 arm and 6.20mmol/L in the D10 arm.Reference Moore and Woollard17
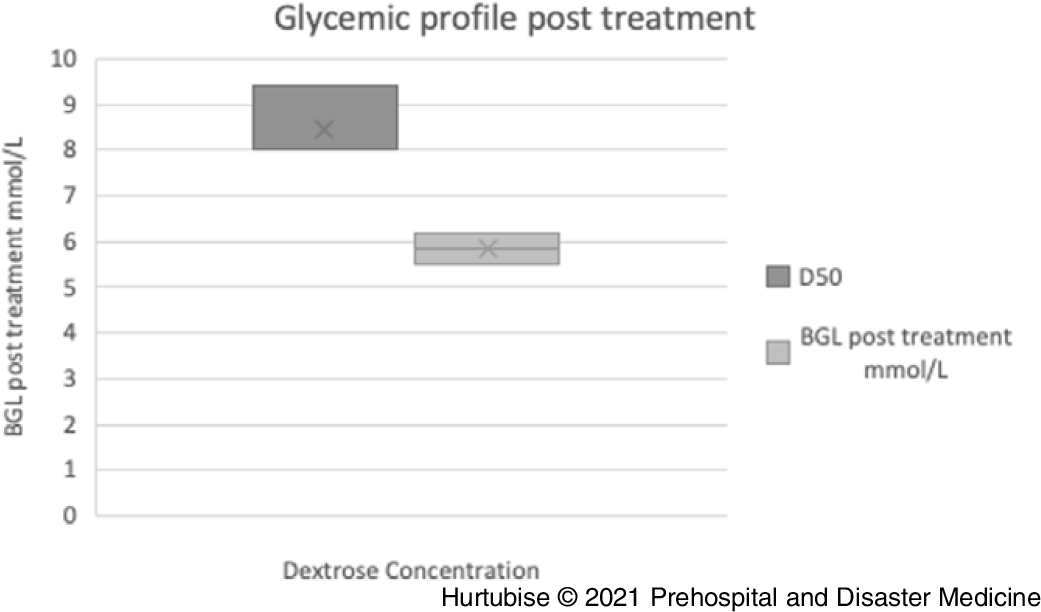
Figure 4. Blood Glucose Post-Treatment.
Abbreviations: BGL, blood glucose level; D50, 50% dextrose.
Need for Subsequent Doses—Evidence from three pooled studies (one RCT and two observational), enrolling 259 patients treated with D50, reported 8.1% (95% CI, 7.9%-8.3%) of patients needed subsequent treatment (Figure 5).Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Mechem24,Reference Weant, Deloney, Elsey, Combs and French25 Results from two observational studies, reporting on 1,032 patients treated with D10, found 19.5% (95% CI, 19.4%-19.6%) required additional D10 treatment.Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Weant, Deloney, Elsey, Combs and French25
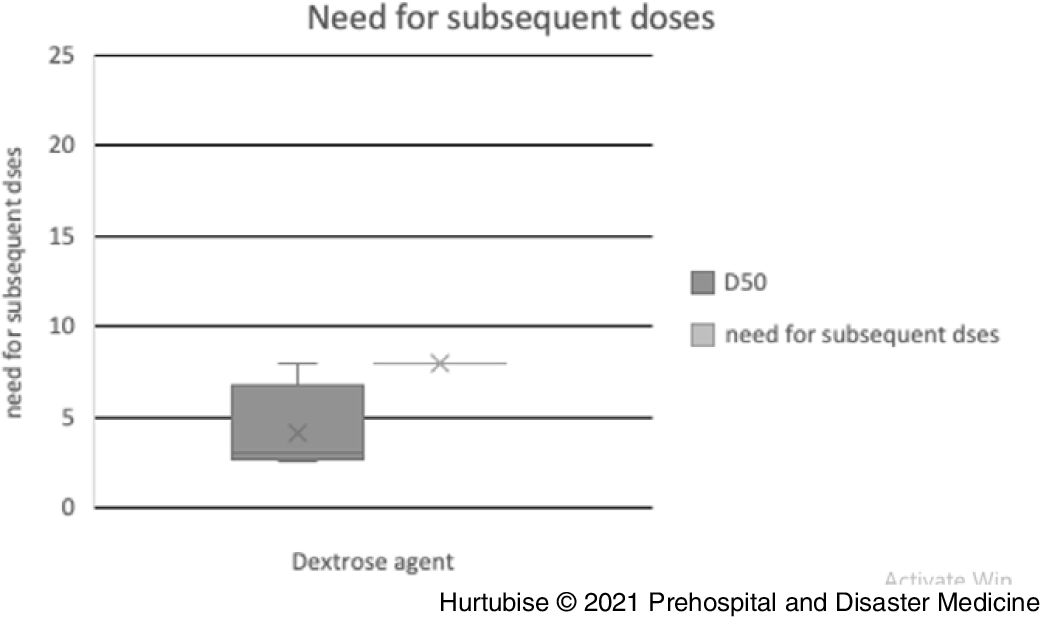
Figure 5. Need for Subsequent Doses.
Abbreviation: D50, 50% dextrose.
Resolution of Hypoglycemia—Evidence from pooled four studies (three RCTs and one observational), enrolling 78 patients treated with D50, reported 98.7% (95% CI, 98.4%-99.0%) resolution of hypoglycemia (Figure 6).Reference Carstens and Sprehn16–Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18,Reference Lerner23 Evidence from two pooled studies (one RCT and one observational), enrolling 896 patients treated with D10, reported 99.2% (95% CI, 99.2%-99.2%) resolution from hypoglycemia.Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Moore and Woollard17 The RCT comparing D50 to D10 reported 100% resolution in both groups: 26 of 26 and 25 of 25 patients, respectively.Reference Moore and Woollard17
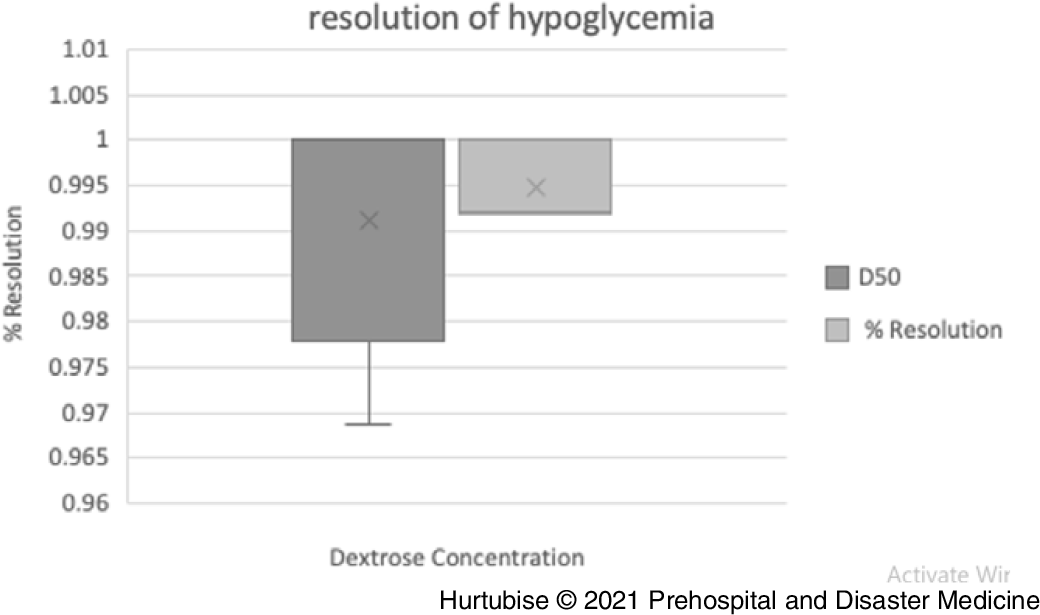
Figure 6. Resolution of Hypoglycemia.
Abbreviation: D50, 50% dextrose.
Time to Resolution of Hypoglycemia—Time to resolution of hypoglycemia was reported on in two RCTs investigating D50 administration in 20 patients.Reference Carstens and Sprehn16,Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18 The RCT by Patrick enrolling 14 patients receiving D50 reported a BGL of approximately12.5mmol/L at the first check five minutes post-treatment.Reference Patrick, Collier, Hepburn, Steedman, Clarke and Robertson18 The RCT by Carstens enrolling six patients receiving D50 reported a BGL of approximately 9.5mmol/L at the first check 10 minutes post-treatment. No data were available for this outcome using D10 treatment.Reference Carstens and Sprehn16
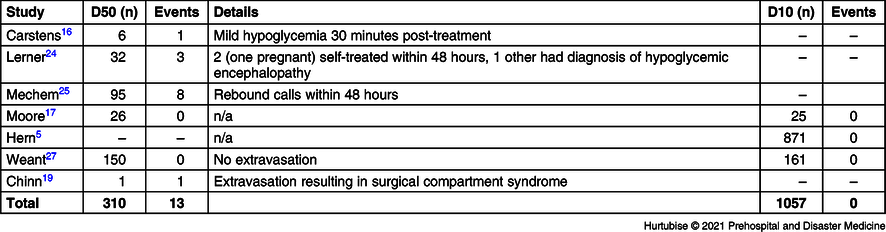
Adverse Events—Seven studies, including 1,367 total patients, investigated adverse events (Table 3 and Table 4). Evidence from six studies (two RCTs and four observational studies), enrolling 310 patients treated with D50, reported a 4.2% (13/310) adverse event rate.Reference Carstens and Sprehn16,Reference Moore and Woollard17,Reference Chinn and Colella19,Reference Lerner23–Reference Weant, Deloney, Elsey, Combs and French25 These consisted of three patients reporting mild hypoglycemia requiring only self-treatment, eight rebound calls to 911 within 48 hours, one patient who did not recover who was later diagnosed in-hospital with encephalopathy as the cause of the hypoglycemia, and one case of extravasation. There were no (0/1,057) adverse events reported in the three studies investigating adverse events for D10 treatment.Reference Hern, Kiefer, Louie, Barger and Alter5,Reference Moore and Woollard17,Reference Weant, Deloney, Elsey, Combs and French25 The RCT reported no adverse events in either treatment group (0/25 D10 group and 0/26 D50 group).Reference Moore and Woollard17 One observational study reported an overall 27.0% (84/311) of patients had hyperglycemia upon hospital arrival with no statistical difference between the D50 and D10 groups. Hyperglycemia had not been considered as an adverse event in this review, but an untoward effect of IV dextrose administration accounted for in the post-treatment BGL results.Reference Weant, Deloney, Elsey, Combs and French25 Mortality had not been listed as an a priori outcome but, of note, the Weant study reported a non-significant difference for in-hospital mortality of 4.7% in the D50 group versus 6.2% in the D10 group (P = .623).Reference Weant, Deloney, Elsey, Combs and French25
Table 3. Results for Each Intervention by Outcome
Abbreviation: BGL, blood glucose level.
Table 4. Adverse Events
Discussion
Summary of Evidence
This review sought to report on the current evidence for the use of D50 and D10 in emergently reversing the immediate impact of hypoglycemia. The aim was to determine if these treatments were equivalent or if one was superior for several patient-related important outcomes. There have been several concerns related to the delivery of the hypertonic D50. A primary concern is neurologic sequala should treatment not be prompt or adequate.4,Reference Jeon, Kim and Kim26 Outcomes of interest were based on the ILCOR guidelines on treatment of hypoglycemia.13 Because of this approach, neurologic sequalae and death were not considered outcomes related to the inclusion criteria. None of the included studies reported on these outcomes. There is the additional concern related to tissue necrosis should the solution become extravasated and post-treatment challenges in blood glucose regulation.Reference Chinn and Colella19,Reference Weant, Deloney, Elsey, Combs and French25 It was for these reasons that there was motivation to identify an efficient evidence-based alternative for patients requiring emergent IV dextrose therapy. The primary outcome of resolution of symptoms was well-reported on and both treatments achieve high levels of success recovering patients to full consciousness. The instances where the cause of failure to rouse the patient were identified; the causes were unrelated to the drug but to the underlying patient’s condition (eg, drugs, alcohol, and serious urinary tract infection).Reference Moore and Woollard17 The time to resolution of symptoms had a notable difference between the two treatments. This difference is thought to be related to the administration modality itself; D10 is typically delivered via an IV drip contrasted to an ampule that is “pushed” delivering the medication intravenously. While this several-minute delay until symptoms are visible diminishing has debatable clinical relevance and long-term outcomes should be formally investigated. However, this slower delivery may allow the clinician greater ability to monitor the patient’s response and titrate the medication to desired effect. The clinicians in the Moore trial were instructed by study protocol to titrate both treatments to desire effect.Reference Moore and Woollard17 This may be the advantage that led to the more desirable post-treatment glycemic profile in the D10 data. Post-treatment hyperglycemia is a documented undesirable effect of emergent dextrose administration which may result in negative neurological impact.Reference Hans and Bonhomme27,Reference Robinson and van Soeren28
Secondary outcomes related to need for subsequent doses and resolution of hypoglycemia had similar results across treatments. Time to resolution of hypoglycemia was only reported for studies investigating D50 where the resolution was reported to be within two minutes. This measure is determined by the interval which the clinicians recheck the BGL and thus may be unreliable. None the less, the patients’ blood glucose meets or exceeds normal levels very quickly post-D50-administration. Notably, there were no adverse events in 1,057 patients reported in the D10 group. There were, however, 13 events considered adverse amongst the patients treated with D50. The most remarkable adverse event was the single case of extravasation resulting in surgical compartment syndrome.Reference Chinn and Colella19 Eleven events were related to rebound hypoglycemia. Three of these cases were considered mild and resulted in self-treatment; two of these three patients were pregnant. Pregnancy is an important factor to note when developing treatment guidelines for hypoglycemia, especially when a treat-and-release policy is present in an EMS service. The other eight rebound hypoglycemic events were serious enough to initiate another 911 call within 48 hours. Overall, these events are manageable and infrequent. Procurement of D50 preloaded ampules has been a challenge for some EMS services; D10 may be mixed as needed, circumventing the preload shortage concern.
Limitations
Measures were taken to reduce investigator-level bias, and although recommended methodology were followed, the resulting data had some inherent limitations. Primarily the lack of head-to-head comparisons of the interventions of interest. The RCT by Moore was low risk of bias and well-reported, however it remained under-powered.Reference Moore and Woollard17 Furthermore, the protocol for administration within the Moore trial left room for potential dose inconsistency because both drugs were subjectively titrated to effect.Reference Moore and Woollard17 Two studies (one RCT and one observational) were scored as high risk of bias primarily due to reporting quality. Many (9 of 11) of the included studies had relatively small sample sizes. There is a paucity of current research as much (7 of 11) of the included data were published prior to 2006. A formal meta-analysis was not possible due to the heterogenous nature of the data, especially in terms of dose and drug comparisons.
Conclusions
High and moderate quality evidence was identified with primarily low risk of bias stating that D10 may be equivalent to D50, for the primary outcome and for some outcomes, potentially a more desirable concentration of dextrose for the treatment of emergent hypoglycemia. Because D10 produces a lower post-treatment glycemic profile, it may mitigate patient’s personal challenges moderating blood glucose. No adverse events, including rebound hypoglycemia, were reported with the use of D10. Although symptom response to D10 is delayed by approximately four minutes compared to D50, D10 appears safer and as effective for the critical outcome of returning consciousness. The two case studies, while well-reported, have inherent risks of bias due to design; these inform on extravasation and D40. More research is needed to determine if the response delay has longer term neurological sequalae on patients.
Acknowledgements
This research was supported by Emergency Health Services Nova Scotia and the Dalhousie Department of Emergency Medicine, Division of EMS. The author team would like to thank the following people for their assistance with this project: administrative support was provided by Melissa MacDougall; Health Sciences Librarian Katie McLean assisted with the development of the search strategy; and Advanced Care Paramedic Martin Jung provided German language translation. The results were presented at the National Association of Emergency Medical Services Physicians conference 2020.
Conflicts of interest
The author team has no conflicts to declare.
Supplementary Materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1049023X21001047















