Article contents
23.—The Synthesis of Derivatives of Acenaphthenone
Published online by Cambridge University Press: 14 February 2012
Abstract
Acenaphthenequinone (I) condensed with ethyl cyanoacetate in ethanol to give the unsaturated ester (IIIb) while condensation with malonic acid in toluene in the presence of diethylamine gave the hydroxy acid (IIa). Esterification of this acid gave an ester (IIb) which could also be obtained by condensation of acenaphthenequinone
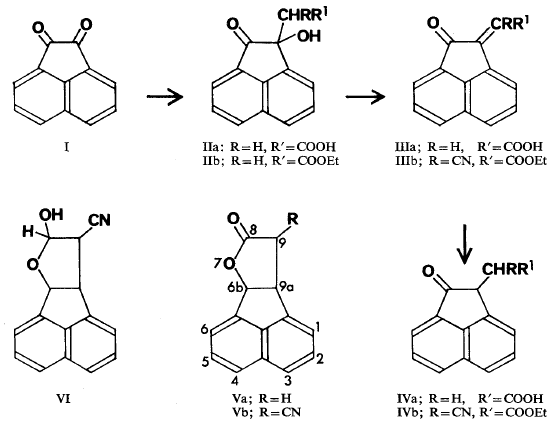
with ethyl hydrogen malonate. The acid (IIa) on dehydration gave 2-oxo-Δ1,α-acenaphtheneacetic acid (IIIa) of m.p. 230°C. This result seems to be in conflict with that of Rodionov and Federova (1950) who reported a m.p. of 160° for the acid (IIIa) which they obtained directly by condensation of acenaphthenequinone with malonic acid in ethanol in the presence of ammonia. Our efforts to repeat their result gave only impure polymeric material and it seems unlikely, therefore, that their product was simply the geometric isomer of our acid.
- Type
- Research Article
- Information
- Proceedings of the Royal Society of Edinburgh Section A: Mathematics , Volume 71 , Issue 4 , 1974 , pp. 279 - 283
- Copyright
- Copyright © Royal Society of Edinburgh 1974
References
References to Literature
- 1
- Cited by




