Article contents
XIV.—The Thermal Equilibrium between Ethylene, Iodine, and Ethylene Di-iodide
Published online by Cambridge University Press: 15 September 2014
Summary
The pressure of ethylene in equilibrium with solid iodine and solid ethylene di-iodide was measured at temperatures between 10°C and 65°C.
The vapour pressure of undissociated ethylene di-iodide was measured at four temperatures between 15°C and 55° C.
From these results and the known vapour pressure of iodine equilibrium constants 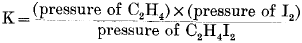 were calculated.
were calculated.
Some experiments were made on the rate of formation and decomposition of ethylene di-iodide.
We desire to thank Professor Sir James Walker and Professor Kendall for their interest in our work, and Imperial Chemical Industries, Ltd., for a grant towards the cost of apparatus.
- Type
- Proceedings
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1930
- 1
- Cited by




