Introduction
Converging lines of genetic, epidemiological and clinical evidence indicate that inflammatory pathways are altered in schizophrenia. Over 20 epidemiological studies show that people with a history of infection or autoimmune diseases have an increased risk of schizophrenia (Benros et al., Reference Benros, Nielsen, Nordentoft, Eaton, Dalton and Mortensen2011, Reference Benros, Eaton and Mortensen2014; Khandaker et al., Reference Khandaker, Zimbron, Dalman, Lewis and Jones2012, Reference Khandaker, Zimbron, Lewis and Jones2013; Miller et al., Reference Miller, Graham, Bodenheimer, Culpepper, Waller and Buckley2013). Variants in genes of the immune pathways have also been associated with an increased risk of schizophrenia (Stefansson et al., Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietiläinen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Børglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Böttcher, Olesen, Breuer, Möller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Réthelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jönsson, Terenius, Agartz, Petursson, Nöthen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009). Moreover, the largest genome-wide association study in schizophrenia to date found a highly significant association between risk of schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics, 2014) and a locus linked to the major immunohistocompatibility complex with subsequent work implicating microglial and complement activation in this pathway (Sekar et al., Reference Sekar, Bialas, de Rivera, Davis, Hammond, Kamitaki, Tooley, Presumey, Baum, Van Doren, Genovese, Rose, Handsaker, Daly, Carroll, Stevens and McCarroll2016). Similarly, elevated levels of a number of immune markers have been observed in schizophrenia (Tourjman et al., Reference Tourjman, Kouassi, Koue, Rocchetti, Fortin-Fournier, Fusar-Poli and Potvin2013). Studies have repeatedly (although not invariably) shown that patients with schizophrenia have increased serum concentrations of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α (Upthegrove et al., Reference Upthegrove, Manzanares-Teson and Barnes2014; Dickerson et al., Reference Dickerson, Stallings, Origoni, Schroeder, Katsafanas, Schweinfurth, Savage, Khushalani and Yolken2016). Meta-analyses show that these are elevated in medication-naïve first-episode patients (Upthegrove et al., Reference Upthegrove, Manzanares-Teson and Barnes2014) and in later stages of illness (Dickerson et al., Reference Dickerson, Stallings, Origoni, Schroeder, Katsafanas, Schweinfurth, Savage, Khushalani and Yolken2016), with large effect sizes (e.g. Hedge's g > 2.2 for IL-6 and >1.1 for IL-1β) (Upthegrove et al., Reference Upthegrove, Manzanares-Teson and Barnes2014). Moreover, studies of cerebrospinal fluid (CSF) have shown increased levels of pro-inflammatory markers in schizophrenia patients when compared with healthy controls, including IL-1β, IL-6 and S100B (Schmitt et al., Reference Schmitt, Bertsch, Henning, Tost, Klimke, Henn and Falkai2005; Soderlund et al., Reference Soderlund, Schroder, Nordin, Samuelsson, Walther-Jallow, Karlsson, Erhardt and Engberg2009; Sasayama et al., Reference Sasayama, Hattori, Wakabayashi, Teraishi, Hori, Ota, Yoshida, Arima, Higuchi, Amano and Kunugi2013; Schwieler et al., Reference Schwieler, Larsson, Skogh, Kegel, Orhan, Abdelmoaty, Finn, Bhat, Samuelsson, Lundberg, Dahl, Sellgren, Schuppe-Koistinen, Svensson, Erhardt and Engberg2015). While there is some evidence for increased inflammatory markers in blood and CSF in schizophrenia, this cannot be taken to suggest neuro-inflammation. The evidence for increased cerebral activation of the immune system is scarce. Post-mortem studies have demonstrated elevated markers for microglia, and morphological changes indicating microglial activation in schizophrenia patients (Bayer et al., Reference Bayer, Buslei, Havas and Falkai1999; Radewicz et al., Reference Radewicz, Garey, Gentleman and Reynolds2000; Trepanier et al., Reference Trepanier, Hopperton, Mizrahi, Mechawar and Bazinet2016) although this was not seen in all brain regions (Steiner et al., Reference Steiner, Mawrin, Ziegeler, Bielau, Ullrich, Bernstein and Bogerts2006) and there are still a large number of null studies (Trepanier et al., Reference Trepanier, Hopperton, Mizrahi, Mechawar and Bazinet2016). However, a recent meta-analysis of post-mortem studies by van Kesteren et al. confirmed overall increased microglia density in schizophrenia, together with increased concentrations of pro-inflammatory proteins (van Kesteren et al., Reference van Kesteren, Gremmels, de Witte, Hol, Van Gool, Falkai, Kahn and Sommer2017).
Microglia are the resident immune cells of the central nervous system and act as major mediators of neuroinflammation. In the healthy brain, microglia retain a ‘quiescent’ phenotype where processes extend through the local environment to detect context-specific changes. In this stage, microglia cells produce neurotrophic factors, provide axonal guidance and regulate local cell proliferation. However, in response to inflammatory stimuli, the cells become activated, undergoing morphological changes and releasing pro-inflammatory cytokines. A number of risk factors for schizophrenia, notably pre-natal infection and psychosocial stress, are known to induce microglial activation (Juckel et al., Reference Juckel, Manitz, Brune, Friebe, Heneka and Wolf2011; Calcia et al., Reference Calcia, Bonsall, Bloomfield, Selvaraj, Barichello and Howes2016). When microglia are activated, it increases the expression of the 18-kDa translocator protein (TSPO) (Cosenza-Nashat et al., Reference Cosenza-Nashat, Zhao, Suh, Morgan, Natividad, Morgello and Lee2009). TSPO can be measured in vivo with positron emission tomography (PET) radiotracers and so far a number of PET studies have investigated microglia activation in schizophrenia-spectrum disorders. However, findings have been inconsistent and so far they have only been partially reviewed quantitatively (Plaven-Sigray et al., Reference Plaven-Sigray, Matheson, Collste, Ashok, Coughlin, Mizrahi, Pomper, Rusjan, Veronese, Wang and Cervenka2018). We therefore aimed to synthesize PET imaging findings of microglial activation in patients with schizophrenia-spectrum disorders and healthy controls, and to discuss the implications of these findings in relation to both the pathophysiology of the disorder and drug development efforts.
Methods
Data source and study selection
The entire PubMed, EMBASE and PsycINFO databases were searched to identify manuscripts published from inception date until 12 January 2018. To be included in the meta-analysis, a study needed to report in vivo TSPO PET imaging data in patients with schizophrenia-spectrum disorders and in a healthy control group. All studies needed to report the mean and standard deviations for both groups (see Fig. 1).
Fig. 1. Flowchart showing the inclusion of studies for the meta-analysis.
Data extraction
The main outcome measure was the difference in the TSPO imaging index between patients with schizophrenia-spectrum disorders and healthy controls. For all studies, we extracted the following variables: authors, year of publication, subject characteristics for the patient and healthy control group (group size, age, sex, diagnosis, duration of illness, antipsychotic medication, psychopathology rating scale scores), imaging characteristics (method, radiotracer) and modelling method. The estimation of pooled standard deviation was performed using the statstodo software (http://statstodo.com/ComMeans_Pgm.php). In order to extract data from studies where data were available only in a plot format, we have used the plot digitiser software (http://plotdigitizer.sourceforge.net/).
Data analysis
The main outcome measure was the effect size determined using the TSPO tracer measure and quantified by either BPND, BP−P or V T in the total grey matter in patients with schizophrenia-spectrum disorders and healthy controls using a random-effects model. A minimum of three studies were required to run a meta-analysis. The group mean and error measures were not reported by Banati and Hickie (Reference Banati and Hickie2009), and although we requested the data from the authors, we were unable to obtain them. Bloomfield et al. (Reference Bloomfield, Howes, Turkheimer, Selvaraj and Veronese2016a, Reference Bloomfield, Selvaraj, Veronese, Rizzo, Bertoldo, Owen, Bloomfield, Bonoldi, Kalk, Turkheimer, McGuire, de Paola and Howes2016b) reported the data in both V T and distribution volume ratio (DVR) (using a multivariate analysis approach, where V T in whole brain or cerebellum was used, along with age and TSPO genotype, as covariates in an analysis of covariance producing a marginal mean). The DVR method used in this study is not equivalent to the measures used in the other studies. Also Ottoy et al. (Reference Ottoy, De Picker, Verhaeghe, Deleye, Wyffels, Kosten, Sabbe, Coppens, Timmers, van Nueten, Ceyssens, Stroobants, Morrens and Staelens2018) reported V T values only accounting for the vascular component (2TCM-1K) (Ottoy et al., Reference Ottoy, De Picker, Verhaeghe, Deleye, Wyffels, Kosten, Sabbe, Coppens, Timmers, van Nueten, Ceyssens, Stroobants, Morrens and Staelens2018). In view of this, we requested the data for this meta-analysis and both authors provided the V T values not accounting for the vascular component, determined in the same way as other studies (i.e. not covaried for age and gender). Therefore, the main analysis of V T values included six studies using the 2TCM model.
A genetic variant at rs6971 in the TSPO gene, causing a non-conservative amino acid substitution, has been found to affect the binding of some TSPO PET tracers (Owen et al., Reference Owen, Yeo, Gunn, Song, Wadsworth, Wadsworth, Lewis, Rhodes, Pulford, Bennacef, Parker, StJean, Cardon, Mooser, Matthews, Rabiner and Rubio2012). Subjects who are homozygotes (LL) have low-affinity binding and have negligible TSPO binding in vivo. Those who are heterozygotes (HL) express both mixed affinity for TSPO (MAB), while those without the polymorphism (HH) have high-affinity binding (HAB) for TSPO (Guo et al., Reference Guo, Owen, Rabiner, Turkheimer and Gunn2012). As on average MABs and HABs have a 22% difference in TSPO binding (Kreisl et al., Reference Kreisl, Jenko, Hines, Lyoo, Corona, Morse, Zoghbi, Hyde, Kleinman, Pike, McMahon and Innis2013), we extracted the data for patients who are HABs and MABs separately to explore the effect of genotype in a sub-analysis.
Publication bias was assessed using visual inspection of funnel plots as well as regression test. Where potential publication bias was suspected, trim and fill analysis was conducted to correct for putatively missing studies. Heterogeneity was estimated using the I 2 value (I 2 values <50% indicate low-to-moderate heterogeneity, whereas I 2 >50% indicate moderate-to-high heterogeneity). Leave-one-out sensitivity analyses were conducted to investigate the potential effect of an individual study on the outcome measure. A p value <0.05 (two-tailed) was taken as a significance level. The statistical analysis of the extracted data was conducted using the R statistical programming language version 3.2.2 with the ‘metafor’ package.
Search strategy
The PubMed, EMBASE and PsycINFO databases were searched without language restrictions. The electronic search using EMBASE and PsycINFO were carried out together using Ovid. The following keywords were used: ‘(Positron Emission Tomography OR PET OR Single photon emission tomography OR SPET OR Single Photon Emission Computed Tomography OR SPECT) AND (schizophrenia OR schizophreniform OR psychosis) AND (microglia* OR microglia* activation OR TSPO OR Translocator protein OR peripheral benzodiazepine receptor OR peripheral benzodiazepine binding site)’. Review papers were also screened to search for additional studies.
Inclusion and exclusion criteria
The inclusion criteria were: original studies in (1) patients with a diagnosis of schizophrenia or related psychotic diagnoses (including schizophreniform disorder; psychotic disorder not otherwise specified, brief psychosis), (2) reporting PET measures using a TSPO-specific ligand and (3) reporting data for the whole grey matter or grey matter regions. Studies that did not have a control group were excluded. Where there was sample overlap between studies, we included the largest one and excluded the other to avoid double counting.
Outcome measures
The primary outcome measure was the effect size for the difference in TSPO PET measure in total grey matter between patients with schizophrenia-spectrum disorders and healthy controls. Where several studies only reported values for grey matter sub-regions, we averaged the grey matter regions to estimate the value for the whole grey matter. The PET studies predominantly reported the outcome either as binding potential (BP) or volume of distribution (V T). As these give different information, we conducted separate meta-analyses of these outcomes. The studies that used BP as an outcome measure have used either values obtained using microparameters from Simplified Reference Tissue Model (Holmes et al., Reference Holmes, Hinz, Drake, Gregory, Conen, Matthews, Anton-Rodriguez, Gerhard and Talbot2016; van der Doef et al., Reference van der Doef, de Witte, Sutterland, Jobse, Yaqub, Boellaard, de Haan, Eriksson, Lammertsma, Kahn and van Berckel2016; Di Biase et al., Reference Di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, McGorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017), but van Berckel et al. (Reference van Berckel, Bossong, Boellaard, Kloet, Schuitemaker, Caspers, Luurtsema, Windhorst, Cahn, Lammertsma and Kahn2008) and Doorduin et al. (Reference Doorduin, de Vries, Willemsen, de Groot, Dierckx and Klein2009) have calculated BP using microparameters derived from 2TCM model. Despite this difference, the results are comparable as reviewed in PET receptor imaging consensus (Innis et al., Reference Innis, Cunningham, Delforge, Fujita, Gjedde, Gunn, Holden, Houle, Huang, Ichise, Iida, Ito, Kimura, Koeppe, Knudsen, Knuuti, Lammertsma, Laruelle, Logan, Maguire, Mintun, Morris, Parsey, Price, Slifstein, Sossi, Suhara, Votaw, Wong and Carson2007).
Results
TSPO binding
There were a total of 12 studies measuring TSPO tracer binding in 190 patients with schizophrenia-spectrum disorders and 200 healthy controls. Five of these studies were in chronic patients, seven in patients within the first 5 years of diagnosis, and two studies also included subjects at ultra-high risk for psychosis. See Table 1 for a summary of sample and study method characteristics. The rationale behind individual study exclusion is documented in supplementary information.
Table 1. Subject and methodological characteristics of the in vivo imaging studies of TSPO binding in schizophrenia compared with healthy controls (BP = 6; V T = 6)

Studies reporting outcome as BP
Six studies reported outcome measures as BP. Our results showed that BP was significantly elevated in patients with schizophrenia when compared with healthy controls with an effect size of 0.31 [Hedge's g = 0.31; z = 2.1; p = 0.03; 95% confidence interval (CI) 0.02–0.6] (Fig. 2). The I 2 test revealed low heterogeneity (I 2 = 0.58%; 95% CI 0–79%). Visual inspection of the funnel plot suggested asymmetry (online Supplementary Fig. S1). The regression test was significant (t = 4.5; df = 4; p = 0.01). The trim and fill analysis showed three putatively missing studies on the left side. The results were not significant after correcting for these studies (Hedge's g = 0.13; z = 0.96; p = 0.34; 95% CI −0.14 to 0.4). The results were significant in two out of six in the leave-one-out analysis, with effect sizes varying from 0.27 to 0.47. Five out of the six studies used the [11C]-PK11195 ligand, with the sub-analysis of these studies revealing a significant elevation of BP in patients with schizophrenia with an effect size of 0.35 (CI 0.01–0.7; p = 0.046) (online Supplementary Fig. S2).
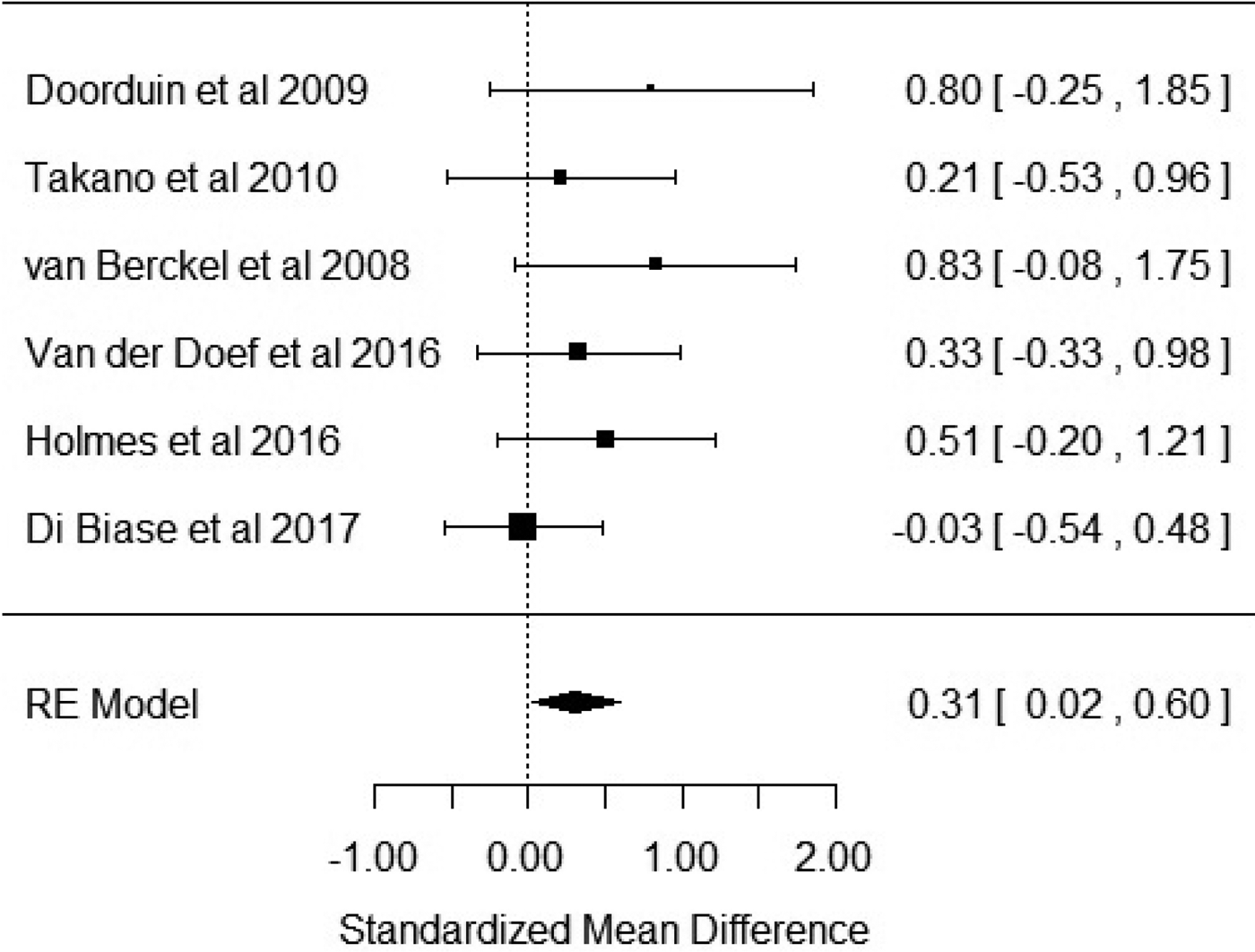
Fig. 2. Forest plot showing the effect sizes for in vivo microglia measures in schizophrenia patients compared with controls as measured by translocator protein binding potential (BP) in total grey matter. There was a significant elevation in schizophrenia with an effect size = 0.31 (p = 0.03).
Studies reporting the outcome as volume of distribution (V T)
Six studies reported the outcome measure as V T. Figure 3 shows that there was no difference in V T in patients with schizophrenia when compared with healthy controls (Hedge's g = −0.22; p = 0.296; CI −0.64 to 0.19). The I 2 test revealed moderate–high heterogeneity (I 2 = 53%; 95% CI 0–92%). Visual inspection of funnel plot suggested asymmetry (online Supplementary Fig. S3). However, the regression test was not significant (t = −0.73; df = 4; p = 0.5). Trim and fill analysis showed two missing studies in the right side. The effect sizes varied from −0.08 to −0.37 in the leave-one-out analysis. We extracted data for high- (HABs) and mixed-affinity binders (MABs) separately from these studies to conduct a sub-analysis stratified by genotype. Bloomfield et al. reported only one patient who was a MAB, precluding accurate estimation of the effect size. Thus, this study was not included in the sub-analysis of MAB subjects (Bloomfield et al., Reference Bloomfield, Selvaraj, Veronese, Rizzo, Bertoldo, Owen, Bloomfield, Bonoldi, Kalk, Turkheimer, McGuire, de Paola and Howes2016b). There was no significant difference between patients and controls in the high-affinity binder sub-analyses (effect size −0.27; p = 0.19; CI −0.68 to 0.13). However, there was a significant difference in the MAB (effect size −0.56; p = 0.03; CI −1.08 to −0.03).

Fig. 3. Forest plot showing effect sizes for in vivo microglia measures in schizophrenia patients compared with controls as measured by volume of distribution of translocator radiotracer (V T) in total grey matter. There were no significant changes in patients compared with controls (effect size = −0.22, p = 0.296).
Discussion
Our main findings are that TSPO PET tracer binding is significantly elevated in patients with schizophrenia relative to controls when BP is used as an outcome measure, with a small-to-moderate effect size (Hedge's g = 0.31; p = 0.03), but there is no significant difference when the tracer volume of distribution (V T) is used as the outcome measure (Hedge's g = −0.22; p = 0.296). In the following section, we consider methodological factors and the implications of our findings.
Methodological considerations
We identified potential publication bias for both outcome measures, and in the case of BP meta-analysis, the results were no longer significant when adjusted for putative missing studies, which if present could potentially affect our findings. Heterogeneity was low for those studies that used BP as an outcome measure and moderate to high in those that used V T as an outcome measure. For this meta-analysis, we used a random-effects model, which is robust to between-study variations. We acknowledge that four of the 12 studies used in this meta-analysis analysed patients with schizophreniform disorder, psychotic disorder not otherwise specified, and brief psychosis as well as patients with schizophrenia (Doorduin et al., Reference Doorduin, de Vries, Willemsen, de Groot, Dierckx and Klein2009; van der Doef et al., Reference van der Doef, de Witte, Sutterland, Jobse, Yaqub, Boellaard, de Haan, Eriksson, Lammertsma, Kahn and van Berckel2016; Collste et al., Reference Collste, Plaven-Sigray, Fatouros-Bergman, Victorsson, Schain, Forsberg, Amini, Aeinehband, Erhardt, Halldin, Flyckt, Farde and Cervenka2017). Two of the studies do not provide a break-down of individual patient participant diagnoses, other than to classify patients as presenting with first-episode psychosis (van der Doef et al., Reference van der Doef, de Witte, Sutterland, Jobse, Yaqub, Boellaard, de Haan, Eriksson, Lammertsma, Kahn and van Berckel2016; Hafizi et al., Reference Hafizi, Da Silva, Gerritsen, Kiang, Bagby, Prce, Wilson, Houle, Rusjan and Mizrahi2017), thus up to 52/190 (27%) of patients included in the meta-analysis are defined as presenting with a schizophreniform disorder, rather than a definitive diagnosis of schizophrenia. Therefore, our results could be influenced by the inclusion of other psychotic disorders, although the low heterogeneity observed for the meta-analysis using BP as an outcome measure at least suggest that including individuals with broader psychotic diagnoses alongside schizophrenia did not have a major impact on the results. Five out of the six studies included in the BP meta-analysis used the first-generation tracer [11C]-PK11195, whereas the V T studies used second-generation tracers. Thus, the difference between BP and V T outcomes could reflect tracer differences, as [11C]-PK11195 is known to have low brain penetration and high non-specific binding, which is a significant limitation of this tracer relative to the second-generation tracers (Fujita et al., Reference Fujita, Imaizumi, Zoghbi, Fujimura, Farris, Suhara, Hong, Pike and Innis2008). However, although the outcome of the BP study that used a different tracer ([11C]-DAA1106) is negative, the results using this tracer are not an outlier, suggesting findings may not entirely be accounted for by tracer differences. It has been suggested that TSPO expression may change during the course of the disorder, which could account for the differences in findings between studies (Notter et al., Reference Notter, Coughlin, Gschwind, Weber-Stadlbauer, Wang, Kassiou, Vernon, Benke, Pomper, Sawa and Meyer2018). However, a recent study by Di Biase et al. using BP as an outcome measure showed no differences between at-risk mental state individuals, recent onset schizophrenia and chronic schizophrenia (Di Biase et al., Reference Di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, McGorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017), and our BP and V T meta-analyses both included studies of chronic and recent onset illness, suggesting that this does not clearly explain the differences between our BP and V T findings. Ultimately, longitudinal studies in patients are required to determine if there are changes during the course of the illness. Other differences between the studies using BP and V T, such as differences in tracer and modelling approaches, could contribute to the differences between our BP and V T meta-analytic findings. Another potential issue is that not all studies accounted for a genetic variant at rs6971 in the TSPO gene. Six out of the 12 studies included in this meta-analysis did not report genotyping (van Berckel et al., Reference van Berckel, Bossong, Boellaard, Kloet, Schuitemaker, Caspers, Luurtsema, Windhorst, Cahn, Lammertsma and Kahn2008; Doorduin et al., Reference Doorduin, de Vries, Willemsen, de Groot, Dierckx and Klein2009; Takano et al., Reference Takano, Arakawa, Ito, Tateno, Takahashi, Matsumoto, Okubo and Suhara2010; Holmes et al., Reference Holmes, Hinz, Drake, Gregory, Conen, Matthews, Anton-Rodriguez, Gerhard and Talbot2016; van der Doef et al., Reference van der Doef, de Witte, Sutterland, Jobse, Yaqub, Boellaard, de Haan, Eriksson, Lammertsma, Kahn and van Berckel2016; Di Biase et al., Reference Di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, McGorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017). Out of these six studies, five used the [11C]-PK11195 tracer while one study used the tracer [11C]-DAA1106. However, genotyping has been shown not to be necessary in studies using [11C]-PK11195, as in vitro studies have shown that this tracer binds to a different site on the TSPO to the locus affected by the rs6971 variant and, consequently, there is no difference in affinity to TSPO between high-affinity and low-affinity binders (Owen et al., Reference Owen, Yeo, Gunn, Song, Wadsworth, Wadsworth, Lewis, Rhodes, Pulford, Bennacef, Parker, StJean, Cardon, Mooser, Matthews, Rabiner and Rubio2012). However, our sub-analyses for the other tracers stratified by genotype showed group differences only remained significant in the MAB groups. We caution against overinterpretation of this finding given the small sample, but suggest it warrants further investigation. Another potential methodological limitation is that a number of studies did not account for partial volume effects. However, as brain volumes tend to be reduced in schizophrenia, partial volume effects would not explain the elevation in BP and, if anything, would tend to reduce the effect size, which means our results may underestimate the true effect. Additionally, in those cases where total grey matter TSPO levels were not available, we have averaged the grey matter regions to estimate the value for the whole grey matter. Although this is a common procedure in PET meta-analyses (Howes et al., Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012; Kambeitz et al., Reference Kambeitz, Abi-Dargham, Kapur and Howes2014; Ashok et al., Reference Ashok, Mizuno, Volkow and Howes2017), it can constitute a potential limitation of this study. Finally, most of the studies in this meta-analysis included patients who were being treated with antipsychotics. Preclinical studies in vitro have found antipsychotic treatment to reduce microglial activation (Zheng et al., Reference Zheng, Hwang, Ock, Lee, Lee and Suk2008), although recent in vivo work has found an increase after olanzapine, but a reduction with risperidone (Zhu et al., Reference Zhu, Zheng, Ding, Liu, Zhang, Wu, Guo and Zhao2014; Cotel et al., Reference Cotel, Lenartowicz, Natesan, Modo, Cooper, Williams, Kapur and Vernon2015; Crum et al., Reference Crum, Danckaers, Huysmans, Cotel, Natesan, Modo, Sijbers, Williams, Kapur and Vernon2016). Critically, none of these studies measured TSPO levels, so it remains unknown if antipsychotics alter TSPO expression. Interestingly, both the studies by Holmes et al. and Di Biase et al. showed that unmedicated patients have lower BP when compared with antipsychotic-treated patients and healthy controls (Holmes et al., Reference Holmes, Hinz, Drake, Gregory, Conen, Matthews, Anton-Rodriguez, Gerhard and Talbot2016; Di Biase et al., Reference Di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, McGorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017). However, this analysis was based only on data provided by 12 patients and further work is thus needed to understand whether antipsychotic treatment could have affected our findings.
Interpretation of findings
The non-displaceable BP (BPND) measures the tracer binding in the tissue of interest relative to another brain region selected to have negligible specific binding, to give specific binding in the region of interest (Mintun et al., Reference Mintun, Raichle, Kilbourn, Wooten and Welch1984). An issue for the measurement of BPND is that there is no brain region with negligible TSPO expression, and consequently no ideal reference region for TSPO tracers (Turkheimer et al., Reference Turkheimer, Rizzo, Bloomfield, Howes, Zanotti-Fregonara, Bertoldo and Veronese2015; Bloomfield et al., Reference Bloomfield, Howes, Turkheimer, Selvaraj and Veronese2016a; Narendran and Frankle, Reference Narendran and Frankle2016). Thus the elevation in BP we report could reflect an increase in specific binding and/or a reduction in non-specific binding in grey matter relative to the reference brain tissue. In contrast, the volume of distribution (V T) measures the total amount of tracer in the brain region relative to that in the blood (Innis et al., Reference Innis, Cunningham, Delforge, Fujita, Gjedde, Gunn, Holden, Houle, Huang, Ichise, Iida, Ito, Kimura, Koeppe, Knudsen, Knuuti, Lammertsma, Laruelle, Logan, Maguire, Mintun, Morris, Parsey, Price, Slifstein, Sossi, Suhara, Votaw, Wong and Carson2007). Volume of distribution is generally the preferred method of quantification of PET tracers where there is no reference region but, critically for group comparisons, assumes blood tracer binding is unaltered between groups (Innis et al., Reference Innis, Cunningham, Delforge, Fujita, Gjedde, Gunn, Holden, Houle, Huang, Ichise, Iida, Ito, Kimura, Koeppe, Knudsen, Knuuti, Lammertsma, Laruelle, Logan, Maguire, Mintun, Morris, Parsey, Price, Slifstein, Sossi, Suhara, Votaw, Wong and Carson2007). However, TSPO tracers may bind to a number of sites in the blood, including acute phase and inflammatory plasma proteins, such as α1-acid glycoprotein (AGP) that are known to be elevated in schizophrenia (Telford et al., Reference Telford, Bones, McManus, Saldova, Manning, Doherty, Leweke, Rothermundt, Guest, Rahmoune, Bahn and Rudd2012; Tourjman et al., Reference Tourjman, Kouassi, Koue, Rocchetti, Fortin-Fournier, Fusar-Poli and Potvin2013). As TSPO tracers bind to AGP (Lockhart et al., Reference Lockhart, Davis, Matthews, Rahmoune, Hong, Gee, Earnshaw and Brown2003), there is the potential for a systematic bias between patients and controls that could affect the quantification of V T, and potentially mask an elevation in the patients’ brain as compared with controls. There is inconsistency in findings in schizophrenia, with one study showing elevation in the plasma concentration levels of [11C]-PBR28 in patients with schizophrenia relative to controls (Bloomfield et al., Reference Bloomfield, Howes, Turkheimer, Selvaraj and Veronese2016a), while two others using [18F]-FEPPA and [11C]-PBR28 show no differences in plasma concentration levels (Hafizi et al., Reference Hafizi, Tseng, Rao, Selvanathan, Kenk, Bazinet, Suridjan, Wilson, Meyer, Remington, Houle, Rusjan and Mizrahi2016; Collste et al., Reference Collste, Plaven-Sigray, Fatouros-Bergman, Victorsson, Schain, Forsberg, Amini, Aeinehband, Erhardt, Halldin, Flyckt, Farde and Cervenka2017). There is also evidence for reduced levels of binding of TSPO tracers to TSPO (previously known as the peripheral benzodiazepine receptor) on platelets in schizophrenia, with reductions of ~30% reported in some studies (Gavish et al., Reference Gavish, Weizman, Karp, Tyano and Tanne1986; Weizman et al., Reference Weizman, Tanne, Karp, Tyano and Gavish1986), although potentially only in certain sub-types of schizophrenia (Wodarz et al., Reference Wodarz, Rothenhofer, Fischer, Stober, Kiehl, Jungkunz, Riederer and Klein1998). Thus, it is possible that changes in either plasma protein binding and/or platelet binding could systematically affect V T values in the disorder, but it should be recognized that there is no direct evidence that plasma binding alters V T values in humans (Cumming et al., Reference Cumming, Burgher, Patkar, Breakspear, Vasdev, Thomas, Liu and Banati2018). Importantly, in a recent study where protein binding was measured, no significant group differences between drug-naive first-episode psychosis patients and healthy controls were observed, suggesting that V T changes are not explained by an effect of protein binding (Collste et al., Reference Collste, Plaven-Sigray, Fatouros-Bergman, Victorsson, Schain, Forsberg, Amini, Aeinehband, Erhardt, Halldin, Flyckt, Farde and Cervenka2017). Further studies are thus needed to clarify if there is an impact of this on the measurement of V T in schizophrenia. Nevertheless, while this is a potential concern for studies using V T as the outcome measure, blood binding should not affect the studies that use a ratio approach, as these methods report tracer uptake relative to another brain region rather than blood. Furthermore, TSPO is expressed on the endothelial cells of brain blood vessels as well as on the outer layer of mitochondria in microglia (Rizzo et al., Reference Rizzo, Veronese, Tonietto, Zanotti-Fregonara, Turkheimer and Bertoldo2014), and both can be accounted for in the PET analysis (Turkheimer et al., Reference Turkheimer, Rizzo, Bloomfield, Howes, Zanotti-Fregonara, Bertoldo and Veronese2015). Few of the studies included in this meta-analysis have accounted for endothelial binding. In view of this, in our meta-analysis, we have included the results not accounting for this compartment. A separate meta-analysis was conducted including the data accounting for the endothelial binding and the results were very similar suggesting this is unlikely to be a major influence on our findings.
Taken together, our meta-analytic findings suggest an elevation in TSPO tracer binding in total grey matter relative to other brain tissue, but not relative to blood, with the caveat that the relative increase is largely based on studies using PK11195, which has a lower specific signal. Thus, this could reflect an increase in TSPO in grey matter or a reduction in TSPO in the reference region, or altered non-specific binding in the brain (Cumming et al., Reference Cumming, Burgher, Patkar, Breakspear, Vasdev, Thomas, Liu and Banati2018). It should also be noted that reductions in TSPO have been reported in some pro-inflammatory states (Narayan et al., Reference Narayan, Mandhair, Smyth, Dakin, Kiriakidis, Wells, Owen, Sabokbar and Taylor2017). Finally, TSPO may also be expressed on astrocytes (Cosenza-Nashat et al., Reference Cosenza-Nashat, Zhao, Suh, Morgan, Natividad, Morgello and Lee2009). While altered TSPO expression on astrocytes may contribute to the differences, the post-mortem findings of unaltered astrocytic but elevated microglial markers (Trepanier et al., Reference Trepanier, Hopperton, Mizrahi, Mechawar and Bazinet2016), including elevated TSPO binding (Kreisl et al., Reference Kreisl, Jenko, Hines, Lyoo, Corona, Morse, Zoghbi, Hyde, Kleinman, Pike, McMahon and Innis2013), are more suggestive of a microglia activity increase in schizophrenia. However, until further work has been conducted to determine if changes in translocator protein expression in schizophrenia are specific to microglia, conclusions about the specificity of changes to microglia should be treated cautiously.
Implications of our findings and future directions
Our different findings depending on the outcome measure used point towards the existence of potential methodological problems in TSPO imaging, raising questions over the interpretation of the elevation in grey matter TSPO binding relative to a reference region in patients with schizophrenia when compared with healthy controls. A recent individual participant data meta-analysis of second-generation radioligand studies, with V T as the outcome measure, showed a reduction in V T in patients relative to healthy controls (Plaven-Sigray et al., Reference Plaven-Sigray, Matheson, Collste, Ashok, Coughlin, Mizrahi, Pomper, Rusjan, Veronese, Wang and Cervenka2018). Although these results seem to be in contrast to the absence of differences in V T in our meta-analysis, our meta-analysis included one more second-generation study showing no differences in V T between schizophrenia patients and healthy controls (Ottoy et al., Reference Ottoy, De Picker, Verhaeghe, Deleye, Wyffels, Kosten, Sabbe, Coppens, Timmers, van Nueten, Ceyssens, Stroobants, Morrens and Staelens2018), which may justify the differences in results between meta-analysis. Ultimately, the definitive test of the importance of TSPO and/or microglia activation in schizophrenia will be to pharmacologically target them with a selective drug or monoclonal antibody combined with PET imaging to confirm target engagement and evaluate the relationship between change in microglial activation and symptomatic improvement. Secondly, it remains to be determined if microglia activity is altered across the different stages of the disorder. Epidemiological and preclinical evidence which indicates that neuroinflammation in utero and early development may predispose to schizophrenia (Meyer, Reference Meyer2013). However, the PET results have been inconsistent, with one study using the tracer [11C]-PBR28 showing an elevation in relative TSPO binding in people at ultra high risk of psychosis using the ratio but not with the V T approach (Bloomfield et al., Reference Bloomfield, Selvaraj, Veronese, Rizzo, Bertoldo, Owen, Bloomfield, Bonoldi, Kalk, Turkheimer, McGuire, de Paola and Howes2016b), while two studies using V T as the outcome measure, using the tracer [18F]-FEPPA and [11C]-PK11195, showed no differences in TSPO binding between healthy controls and in individuals at ultra high risk of psychosis for psychosis (Hafizi et al., Reference Hafizi, Tseng, Rao, Selvanathan, Kenk, Bazinet, Suridjan, Wilson, Meyer, Remington, Houle, Rusjan and Mizrahi2016; Di Biase et al., Reference Di Biase, Zalesky, O'Keefe, Laskaris, Baune, Weickert, Olver, McGorry, Amminger, Nelson, Scott, Hickie, Banati, Turkheimer, Yaqub, Everall, Pantelis and Cropley2017). Unfortunately, there were not sufficient studies to proceed with a sub-analysis of studies conducted in the early phase of illness. Future research should focus on at-risk mental state individuals and early in the development of the illness and use longitudinal designs to determine the role of microglial activity in the different stages of the disorder. Ideally, these studies should be conducted before antipsychotic medication initiation, as it would also help to clarify the potential role of antipsychotics in microglia activity. Interestingly, it has been shown that pro-inflammatory cytokines are elevated in first-episode patients who do not respond to antipsychotic treatment relative to those who do respond (Mondelli et al., Reference Mondelli, Ciufolini, Belvederi Murri, Bonaccorso, Di Forti, Giordano, Marques, Zunszain, Morgan, Murray, Pariante and Dazzan2015), which suggests that increases in microglia activity may be specific to a sub-group of patients, consistent with neurobiological sub-types of schizophrenia (Howes and Kapur, Reference Howes and Kapur2014). Studies that focus on patients that do not respond to conventional antipsychotic medication should be able to shed light on this topic. When interpreting our results is also important to note that this meta-analysis focused on total grey matter TSPO binding, and there was insufficient data for a meta-analysis of specific brain regions. Although TSPO is ubiquitous and expressed across the whole brain, we cannot exclude that microglia activation in schizophrenia is differentially expressed in specific regions within the brain. Indeed, early studies by Doorduin et al. (Reference Doorduin, de Vries, Willemsen, de Groot, Dierckx and Klein2009) and van Berckel et al. (Reference van Berckel, Bossong, Boellaard, Kloet, Schuitemaker, Caspers, Luurtsema, Windhorst, Cahn, Lammertsma and Kahn2008) suggest increased TSPO binding in the medial temporal cortex, and Bloomfield et al. (Reference Bloomfield, Selvaraj, Veronese, Rizzo, Bertoldo, Owen, Bloomfield, Bonoldi, Kalk, Turkheimer, McGuire, de Paola and Howes2016b) using the DVR approach also found evidence of this in people at ultra high risk of psychosis. Finally, future studies are needed to address the methodological issues and sources of variance discussed above. This could be potentially achieved by obtaining individual patient data from each individual study and applying both ratio and volume of distribution models to determine if PET modelling or study differences drive the opposing BP and volume of distribution findings. In addition, we recommend that new studies present results for both modelling approaches so that consistency of findings can be compared and support future meta-analysis, while the role of vascular binding in PET TSPO quantification should be clarified.
Conclusion
In conclusion, there is evidence for a moderate effect size elevation in TSPO tracer binding in grey matter in schizophrenia-spectrum disorders when using BP as an outcome measure, but no changes when V T is the outcome measure used. These results suggest that potential methodological differences between TSPO studies need to be accounted for and addressed in future studies and keep open the discussion over the existence of an increase in microglia activity in patients with schizophrenia-spectrum disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003057.








