Introduction
The need for effective population-wide prevention approaches to depression, including mixed depression and anxiety (Tyrer, Reference Tyrer2001; Das-Munshi et al. Reference Das-Munshi, Goldberg, Bebbington, Bhugra, Brugha, Dewey, Jenkins, Stewart and Prince2008), has been highlighted by the demonstration of undiminished population prevalence rates despite at least a twofold increase in the usage of evidence-based treatments (Brugha et al. Reference Brugha, Bebbington, Singleton, Melzer, Jenkins, Lewis, Farrell, Bhugra, Lee and Meltzer2004; Kessler et al. Reference Kessler, Demler, Frank, Olfson, Pincus, Walters, Wang, Wells and Zaslavsky2005). Evidence of prevention of depression has not been reported in a trial in adults but may be feasible; in a recent meta-analysis synthesizing the results of 19 small prevention trials (Cuijpers et al. Reference Cuijpers, van Straten, Smit, Mihalopoulos and Beekman2008), the mean incidence rate ratio (IRR) of developing a depressive disorder (including postpartum depression) was 0.78 [95% confidence interval (CI) 0.65–0.93], indicating a reduction of the incidence by 22% in experimental compared to control groups.
Prevention can be considered as avoiding future illness in those already at risk although disease free or, more strictly, as avoiding future illness for all the population, not just those at immediate risk (Rose, Reference Rose2001), hence the term universal prevention (Gordon, Reference Gordon1983; Mrazek & Haggerty, Reference Mrazek and Haggerty1994). But how might this be achieved? A highly significant predictor of depression within adulthood is ‘subthreshold’ depression (Judd et al. Reference Judd, Schettler and Akiskal2002; Cuijpers & Smit, Reference Cuijpers and Smit2004). Depression prevention through the extension of cognitive behavioral therapy (CBT) to at-risk adolescents who had symptoms that fell short of the threshold for case depression (Clarke et al. Reference Clarke, Hornbrook, Lynch, Polen, Gale, Beardslee, O'Connor and Seeley2001; Garber et al. Reference Garber, Clarke, Weersing, Beardslee, Brent, Gladstone, DeBar, Lynch, D'Angelo, Hollon, Shamseddeen and Iyengar2009) may be effective because subthreshold levels are highly predictive of depression (Cuijpers et al. Reference Cuijpers, van Straten and Smit2005). It is not known whether such skilful psychological approaches could also benefit a much wider range of people at risk of developing depression if delivered universally across an entire population to adults at all risk levels.
In the UK all infants and all mothers, following childbirth, receive individual care from a specialist community nurse, known as a ‘Health Visitor’ (HV). In addition to supporting infant care, the HV has a role in maternal mental health that should involve establishing a relationship with the mother and the use of interpersonal and communication skills (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b). However, such practitioners are given little more than basic mental health knowledge. Could training HVs in psychological skills benefit women under their care who were not at immediate risk? In this paper we report on the benefits for all non-depressed postnatal women randomized to receive support from primary care nurses with specialized training in psychologically informed approaches to health care, in a large pragmatic cluster randomized controlled and economic evaluation trial.
We have already reported on postnatal women in randomly allocated intervention group (IG) clusters cared for by an HV who had received additional training in postnatal mental health assessment and in one of two psychologically informed approaches that were compared to usual HV care (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a). Clinical assessment and psychologically informed approaches were designed to be offered by intervention group HVs to women scoring positive on the Edinburgh Postnatal Depression Scale (EPDS) (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b) at 8 weeks following childbirth. The EPDS is a widely used self-report measure to identify women at risk of postnatal depression (PND) with good evidence of sensitivity and specificity (Gibson et al. Reference Gibson, Kenzie-McHarg, Shakespeare, Price and Gray2009; Hewitt et al. Reference Hewitt, Gilbody, Brealey, Paulden, Palmer, Mann, Green, Morrell, Barkham, Light and Richards2009) and a score ranging from 0 to 30, consisting of eight depression and two anxiety items. We compared IG care with postnatal care as usual (CAU). We showed that these scoring positive IG women were less likely than CAU women to have later depression, indicated by scoring ⩾12 on a postal EPDS at 6 months postnatally [odds ratio (OR) 0.62, 95% CI 0.04–0.97, unadjusted p=0.036] (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a).
The trial was also designed to assess effectiveness according to severity of depression at baseline (www.controlled-trials.com/ISRCTN92195776). Because outcome data were obtained on all randomized and consenting women, we were able to estimate any effects on women with baseline symptom scores below the 6 weeks postnatal EPDS threshold of ⩾12 (Cox et al. Reference Cox, Holden and Sagovsky1987). Thus, the present paper reports new, unpublished analyses of outcomes from the original treatment trial, principally for women who scored <12 on the postal EPDS at 6 weeks postnatally, using the same trial primary outcome: EPDS ⩾12 at 6 months. First, effects on outcomes at 6, 12 and 18 months postnatally were examined for women with an EPDS<12 at 6 weeks. Second, to test whether additional training of HVs was of benefit to women across a lower range of EPDS-negative scores, we compared the effectiveness in women at the lowest EPDS severity levels (EPDS<6 at 6 weeks) with subthreshold scoring women (6-week EPDS scores from 6 to 11). Third, we examined differences in HV care, including the use of randomly allocated face-to-face administered depression assessment, because such additional practitioner involvement could explain any observed effect on later risk of depression.
Method
The main pragmatic cluster randomized trial design and methods, on which the present analysis is based, are reported in detail elsewhere (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a). The present study involved assessing outcomes for women in 101 primary care practices (clusters) (in one region of England, serving a population of 5.1 million), termed ‘EPDS-negative’, who had a postal EPDS score <12 at 6 weeks postnatally. Following recruitment of participating practices, an independent statistician generated the random allocation sequence using a computer randomization program (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a). Thus, HVs registered to consenting practices were randomized either to continue to provide postnatal CAU or to an experimental IG in which all HVs received additional training in systematic assessment of depressive symptoms, establishing warm, therapeutic relationships, and in one of two distinct experimental psychologically informed approaches. Eligible antenatal women were then recruited if they were registered with participating (and therefore randomized) GP practices, aged ⩾18 years and had no severe or enduring mental health problems. HVs were asked to send these women a research information leaflet and a consent form at 36 weeks of pregnancy or 4 weeks antenatally. Women returned their signed forms to the HVs. Altogether, 4084 (53.4%) of 7649 eligible antenatal women provided written informed consent during 2003–2004. Consenting women were sent a questionnaire at 6 weeks and 6, 12 and 18 months postnatally from the research office. Women who scored ⩾12 on their 6-week postal EPDS were regarded as EPDS positive (n=595 women in 86 clusters) and were excluded from all of the present outcomes analyses. Half of all women in the intervention clusters (including EPDS-negative women) were randomly allocated to complete a face-to-face EPDS 6 weeks postnatally to coincide with usual HV care during an existing HV contact. The intervention cluster HVs, if their clinical assessment indicated that any mother might benefit, could also offer the psychologically informed sessions to which their practice had been randomized.
Identical to the treatment trial protocol (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b), the pretrial determined primary outcome was the proportion of women scoring ⩾12 on the EPDS at 6 months postnatally. We assumed an intra-cluster coefficient (ICC) of 0.006 (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b) and an average cluster of practice size of 25 EPDS-negative women. Thus, 2489 women (1659 IG and 830 CAU) were required to have 80% power at the 5% two-sided level of significance, to detect a 3.5% absolute difference (10% v. 6.5%) in the proportions of 6-week EPDS-negative IG and CAU women scoring ⩾12 on the EPDS at 6 months postnatally. Clusters were allocated for the trial to one of two HV psychologically based approaches (experimental IG) or HV CAU, in a ratio of 1:1:1. The clusters were stratified by the number of expected births per cluster per year, into three groups (<70, 70–100, >100).
The cluster level intervention comprised the package of HV training in assessment of postnatal women and identification of depressive symptoms using the EPDS and additional clinical assessment skills and establishing warm, therapeutic relationships. They were trained to deliver one of two distinct psychologically informed approaches [a cognitive-behavioral approach (CBA) and a person-centered approach (PCA)], delivered at the individual level, with the provision of additional supervisory support for the HVs (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b). The HV-provided psychologically informed session was a one-hour visit, once a week, for a maximum of 8 weeks, commencing around 8 weeks postnatally. Sessions were offered to women who scored ⩾12 on the postal EPDS at 6 weeks and also on a face-to-face EPDS administered at 8 weeks postnatally.
IG HVs received a day of training in the identification of depression using the EPDS and clinical assessment. The group training in the pretrial preparatory phase lasted 8 days in total (including four half-days), focusing on the development of cognitive behavioral or person-centered intervention skills delivered in a group training format. A common element of the training, preceding specific approach-based skills, concerned development of empathic relationships. The training package was followed by two half-day reflective practice sessions and access to regular supervision sessions. Two experienced psychotherapist practitioner trainers delivered the training, which followed a prepared manualized form and was agreed by a training reference group composed of experienced psychotherapy trainers. HVs were asked to tape record their intervention sessions (with the women's consent) so that session treatment fidelity could be checked. From the recorded sessions available it was clear that high levels of adherence were achieved. HVs and women were also asked to complete the Agnew Relationship Measure to measure the therapeutic relationship between them. Further details of the training are available in Morrell et al. (Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b) and in findings to be reported separately.
HVs were blind to all research outcomes. Secondary outcomes were measured in all women who were followed up at 6, 12 and 18 months postnatally by postal questionnaires (including the EPDS at 6 and 12 months only). These included the 12-item Short Form Health Survey (SF-12) and the SF-6D (Ware et al. Reference Ware, Kosinski and Gandek1995), the Clinical Outcomes in Routine Evaluation – Outcome Measure (CORE-OM) scales (Barkham et al. Reference Barkham, Margison, Leach, Lucock, Mellor-Clark, Evans, Benson, Connell, Audin and McGrath2001) and the State–Trait Anxiety Inventory (STAI; Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970). We used the CORE-OM as a measure of global distress as used by psychological therapy services to distinguish between clinical and general populations. We measured postnatal anxiety symptoms using the STAI (Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970). To achieve the required sample size for the 6-month primary outcome, the recruitment phase was extended. However, the follow-up phase was not similarly extended; therefore, all the women for whom a 6-month outcome was available did not have a baby who was 1 year or 18 months old within the follow-up phase. Therefore, fewer questionnaires were administered at these time points. We requested funds to extend the recruitment phase but none were provided.
Ethical approval was received from Trent Region Multi-centre Research Ethics Committee.
Statistical analysis
The primary comparison reported for this analysis was between eligible women in randomized IG practices versus those in CAU randomized practices, with an EPDS score <12 at 6 weeks postnatally in addition to a valid 6-month EPDS outcome score (n=2241 EPDS-negative women in 101 clusters). All analyses were by intention to treat with a p value <0.05 regarded as statistically significant. A marginal generalized linear model (GLM), with coefficients estimated using generalized estimating equations (GEEs), with robust standard errors and an exchangeable autocorrelation matrix in Stata version 11 (StataCorp, 2009) was used to analyze outcomes and allow for the clustered nature of the data, with associated 95% CIs. We fitted simple unadjusted models, and also adjusted outcome comparisons for individual level covariates: living alone, history of PND, presence of stressful life events (Brugha et al. Reference Brugha, Bebbington, Tennant and Hurry1985) and 6-week EPDS score, as the most widely identified significant predictors of PND (O'Hara & Swain, Reference O'Hara and Swain1996). To test whether any intervention effect was confined to those scoring immediately below the EPDS scoring cut-off only, we performed an intervention by baseline (6-week) EPDS score statistical interaction test. This compared women grouped to baseline EPDS scores of 6–11 with women with a baseline EPDS score <6. We compared 6-month outcomes according to whether women were in practices randomized for HVs to provide a face-to-face assessment at 6 weeks plus postal assessment compared to postal EPDS only. Evidence for diminished and differential health benefit over time, between the groups, was tested for in a longitudinal model by looking for evidence of an IG by time interaction. Thus, longitudinal modeling of 6, 12 and 18 months secondary outcomes, also using a marginal GLM with coefficients estimated by GEEs, was performed to examine time effects, group differences and possible time by group interactions on the secondary outcomes. The longitudinal models adjust for clustering of individual responses over time and not by practice. For the primary outcomes, the binary EPDS scores at 6 and 12 months, we used the multiple imputation procedure in STATA version 11 (StataCorp, 2009) to impute missing EPDS outcomes based on 100 imputations using a logistic regression model with 6-week EPDS score, lives alone, history of PND and life events as predictors. The trial is reported according to the CONSORT guidelines for cluster trials (Campbell et al. Reference Campbell, Elbourne and Altman2004).
Results
Figure 1 shows the trial profile. The 6-week questionnaire return rate was 83.7% (3419/4084), of whom 17.4% of women (595/3419) had an EPDS score of ⩾12 and 82.6% (2824/3419) were EPDS-negative. At 6 months, 79.4% (2241/2824) of the EPDS-negative women had returned both 6-week and 6-month questionnaires, 767 in the control (CAU) group and 1474 in the IG. Table 1 shows the baseline and 6-week characteristics of these 2241 new mothers. The observed ICC for the primary outcome, the proportion of women with an EPDS score of ⩾12 at 6 months, in the 100 clusters was 0.001 (95% CI 0.000–0.015).
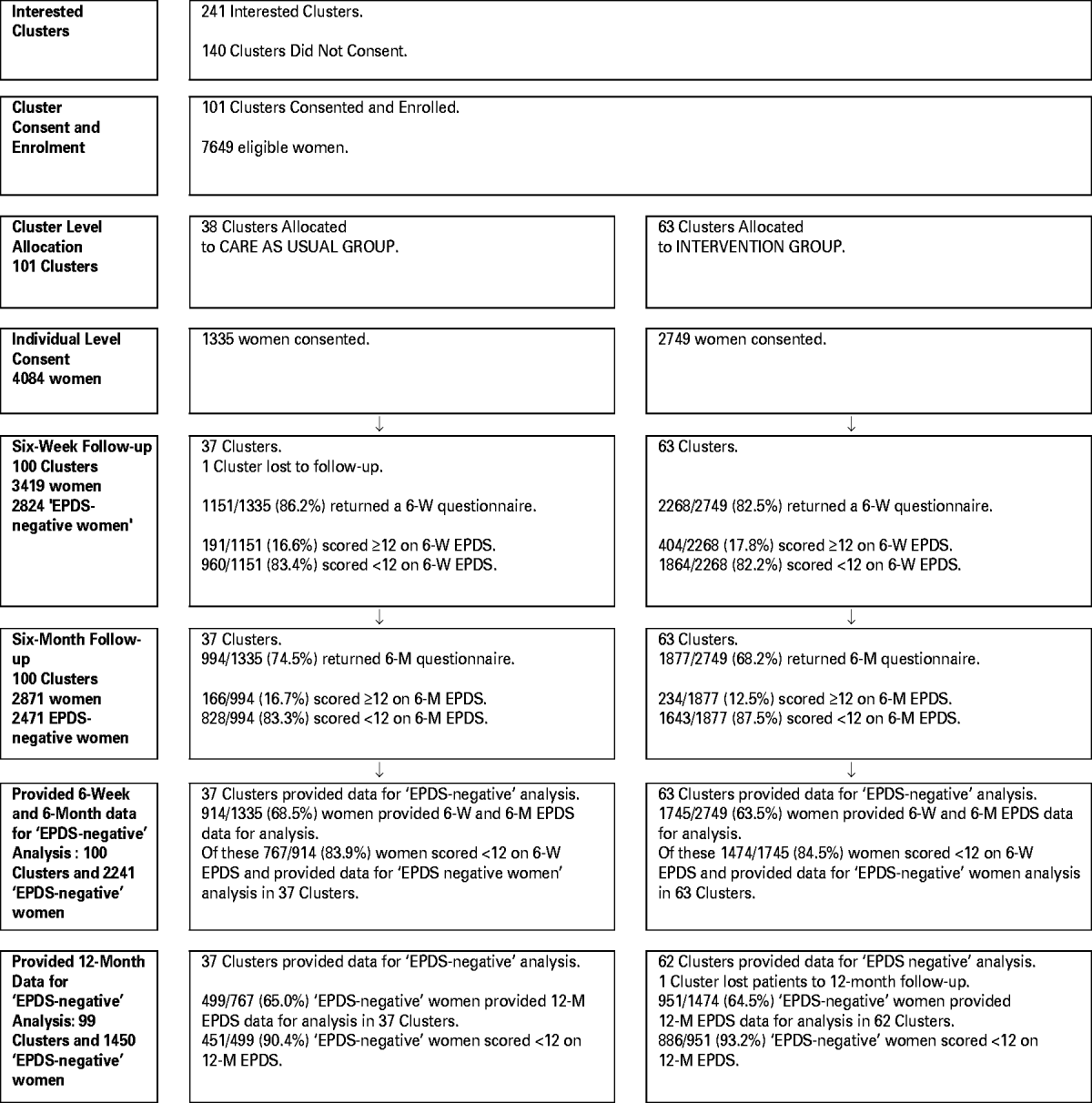
Fig. 1. Trial profile of clusters and participants in the intervention group (IG) and the care as usual (CAU) group for all women and Edinburgh Postnatal Depression Scale (EPDS) negative women.
Table 1. Baseline and 6 weeks characteristics of ‘EPDS-negative women’ by group (n=2241)
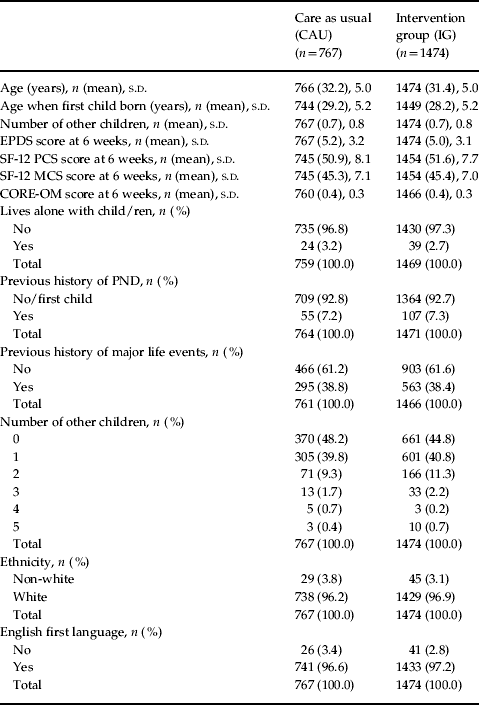
EPDS, Edinburgh Postnatal Depression Scale; SF-12, 12-item Short Form Health Survey; PCS, physical component summary scale; MCS, mental component summary scale; CORE-OM, Clinical Outcomes in Routine Evaluation – Outcome Measure; PND, postnatal depression, s.d., standard deviation.
Better health is represented by a lower score in CORE-OM and EPDS and a higher score in the SF-12 and SF-6D.
As reported previously (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a), Table 2 shows that, at 6 months following childbirth, 83 out of 767 (10.8%) control (CAU) women and 113 of 1474 IG women (7.7%) scored ⩾12 on the EPDS at 6 months, that is an absolute difference of 3.1% (95% CI 0.4–5.5) or a relative difference of 0.68 (95% CI 0.50–0.93, p=0.016). Of the covariates (living alone, previous PND, presence of adverse life events in the past 6 months and 6-week EPDS score), only living alone was not a significant predictor of the EPDS at 6 months. After adjusting for individual-level covariates, the OR for a score of ⩾12 at 6 months was 0.71 (95% CI, 0.53–0.97, p=0.031) for IG women compared with CAU women. Multiple imputation computing for missing 6-month EPDS scores increased the sample size from 2241 to 2786, and the OR using the imputed data, with adjustment for covariates, was similar to the observed data (0.76, 95% CI 0.56–1.03, p=0.073).
Table 2. Primary outcome: proportion of EPDS-negative women with EPDS score ⩾12 at 6 months, by intervention group (IG) or care as usual (CAU) group, comparing EPDS subthreshold and lowest EPDS score subsamples defined according to EPDS score at 6 weeks (n=2241)
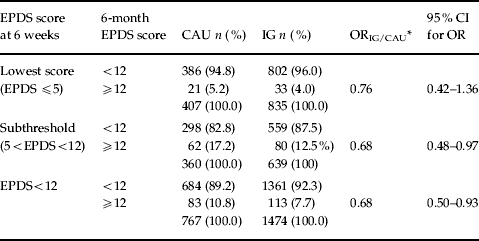
EPDS, Edinburgh Postnatal Depression Scale; OR, odds ratio; CI, confidence interval.
p value for interaction term between lowest score (EPDS ⩽5) and subthreshold (5<EPDS<12) groups=0.782.
* p value for interaction term adjusted for lives alone (yes/no), history of postnatal depression (PND) (yes/no), any life events (yes/no)=0.749.
The EPDS-negative women were divided into two subgroups: a ‘subthreshold’ subgroup scoring between 6 and 11 on the 6-week EPDS (n=999) and a ‘lowest severity’ subgroup scoring 0–5 on the 6-week EPDS (n=1242) based on the approximate median score for the sample. The choice of a cut-point between 5 and 6 on the EPDS was supported by the finding that 55% (1242/2241) of EPDS-negative women scored 0–5. Those in the subthreshold subgroup (score 6–11) did not benefit significantly more from IG membership as defined by the 6-week EPDS score (0–5, compared to 6–11); a significant intervention by subgroup interaction was not found (z=−0.28, p=0.782) (Table 2). The ORs for the effect of HV training in these two EPDS score subgroups scarcely differed (0.76 and 0.69 respectively; Table 2). The results of a similar comparison of low- and high-risk women also showing no difference in effectiveness are available on request.
Among the secondary outcomes of this study, at 6 months the mean EPDS score in EPDS-negative women was 5.4 (s.d=4.5) for CAU women and 4.8 (s.d=4.2) for IG women, a difference of −0.6 (95% CI −0.9 to −0.1, p=0.007), which was statistically significant (p=0.007). This difference remained statistically significant after adjusting for the same 6-week and baseline covariates (p=0.013) (Table 3). A significant difference (p=0.020) in the 6-month CORE-OM total score favoring the IG women (mean=0.38) over CAU women at 6 months was also found (mean=0.43) with other outcome comparisons (SF-12 and State Anxiety) being not significant. A comparison of outcomes for IG women in the two randomly allocated training groups (CBA or PCA) also showed no difference in effectiveness: compared to the control (CAU) EPDS score, at 6 months the mean EPDS score in the CBA group was 4.7 (n=708, s.d=4.2); and in the PCA group it was 4.9 (n=766, s.d=4.3).
Table 3. Six-month secondary outcomes for the ‘EPDS-negative sample’ (n=2241), care as usual (CAU) versus intervention group (IG), unadjusted and adjusted
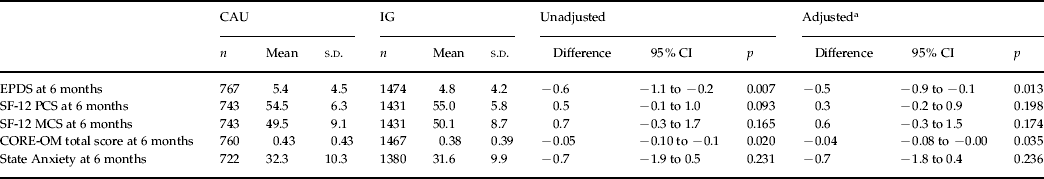
EPDS, Edinburgh Postnatal Depression Scale; SF-12, 12-item Short Form Health Survey; PCS, physical component summary scale; MCS, mental component summary scale; CORE-OM, Clinical Outcomes in Routine Evaluation – Outcome Measure; s.d., standard deviation; CI, confidence interval.
Better health represented by lower score in CORE-OM, EPDS, STATE Anxiety. Better health represented by higher score in SF-12.
a Adjusted for 6-week EPDS score, lives alone, history of postnatal depression (PND) and any life events.
EPDS-negative women in the face-to-face clinical assessment group (n=746) had a lower mean EPDS score at 6 months than women who only received a postal EPDS (n=728; difference 0.68, 95% CI 0.25–1.20, p=0.002); this was of borderline statistical significance following adjustment (p=0.054). When comparing the binary 6-month EPDS outcomes (EPDS score ⩾12), the differences were not statistically significant (unadjusted p=0.126; adjusted p=0.301).
Because of trial funding limitations, fewer postal questionnaires were sent to women and thus returned at 12 and 18 months (n=807) than at 6 months (n=2659). In addition to the 6-week and 6-month returns, 1450 women also completed and returned a postal follow-up at 12 months and 806 at 18 months. At the 12-month follow-up, 9.6% (48/499) of women in the CAU group and 6.8% (65/951) of women in the IG had an EPDS score ⩾12 and an unadjusted OR of 0.68 (95% CI 0.44–1.05, p=0.079). After adjusting for covariates, the OR was 0.74 (95% CI 0.48–1.14, p=0.167). Using a multiple imputation method for computing missing 12-month EPDS scores increased the sample size from 1450 to 2786, and the OR using the imputed data, with adjustment for covariates, was 0.89 (95% CI 0.62–1.28).
Group effects and time effects were found for the EPDS (continuous) outcome. However, after adjusting for baseline covariates, no significant group by time statistical interactions were found on any of the outcome measures [EPDS binary, EPDS continuous, CORE-OM, SF-12 mental component summary scale (MCS), SF-12 physical component summary scale (PCS), State-Anxiety]. Group effects, but not time effects, were found for the CORE-OM and EPDS binary outcomes. A significant time effect only was observed for the two SF-12 outcomes. No group or time effects were observed for the State Anxiety outcome.
Discussion
Our analysis has shown that women who scored below the EPDS threshold at 6 weeks after childbirth were less likely to score above the EPDS threshold at 6 months after childbirth if their HV had undergone training in identifying depressive symptoms, developing therapeutic relationships and providing psychological approaches to managing depression. Furthermore, this effect applied equally across women at all initial levels of risk, not just those close to the above threshold, thus suggesting a universal effect. This effect did not diminish over later assessments at 12 and 18 months following childbirth. How plausible is this effect and can it be explained?
We found that the training was effective in the separately reported treatment trial in EPDS positive (EPDS score ⩾12) women (Morrell et al. Reference Morrell, Slade, Warner, Paley, Dixon, Walters, Brugha, Barkham, Parry and Nicholl2009a), although 61% agreed to, and only 28% of all EPDS positive IG women providing outcome data had completed, HV sessions. In the present study, approximately 1% of IG EPDS-negative women were offered and completed at least one session. So we need to ask why and how was HV training universally effective most notably in the EPDS-negative women? Cross-contamination effects are likely to have been minimized by clustering HVs. One previous primary care trial, using a similarly powered clustered design, in which midwives were trained in a protocol to focus on specific physical and mental health needs of mothers after childbirth, also reported significantly lower EPDS scores at 4 months follow-up in women in IG practices (MacArthur et al. Reference MacArthur, Winter, Bick, Knowles, Lilford, Henderson, Lancashire, Braunholtz and Gee2002). However, there were insufficient baseline data available to identify women with greater depression severity to determine whether effectiveness was confined to a subgroup of likely cases of depression or was preventive across the whole range of baseline severity.
What seems to matter is the difference between being registered with a practice that has adopted the experimental versus the CAU policy. This involves differences in practice environment and culture. More specifically, receiving training and developing the skill to assess mental health and provide psychologically informed sessions is likely to affect the practitioners themselves through increased confidence. It creates a focus of care on the mother's psychological well-being and not just on the physical welfare of the child. Women may benefit from the HVs' enhanced communication skills and knowing that emotional issues are open for discussion if needed, without the possibly stigmatizing requirement to refer to a different profession or service. We were able to show evidence in the women who were below threshold at 6 weeks of a positive effect of being randomly assigned to receive a face-to-face EPDS administration and clinical assessment, when compared with a research office postally administered EPDS questionnaire only. As reported separately, empirical support for change in HV activity was shown in the resource use logs of these lower-risk women (Brugha et al. Reference Brugha, Morrell, Slade and Dixon2009). As the EPDS score increased, in the IGs, a clear increase in the number of visits was seen (Brugha et al. Reference Brugha, Morrell, Slade and Dixon2009), which seemed to be due to a greater recognition of the need for professional input relating to PND by IG teams; whereas concurrently, there were fewer visits by intervention HVs relating to the mothers with lower EPDS scores, presumably because visits are judged to be unnecessary.
The question remains unanswered: how did intervention cluster women benefit without formal psychological therapy sessions? The purpose of training was also to establish a high-quality, warm, therapeutic relationship with the women (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b). Concepts of social support have been suggested to explain the common underlying mechanisms in the effectiveness of cognitive behavioral, interpersonal and other therapies (Brugha, Reference Brugha and Brugha1995). Tailoring to need and availability of support from a trained professional could potentially influence perception of available support, which may be the key parameter, rather than objective support. The value of tailoring and flexible care has certainly been suggested by MacArthur et al. (Reference MacArthur, Winter, Bick, Knowles, Lilford, Henderson, Lancashire, Braunholtz and Gee2002), but in that study women were provided care from midwives and followed only to 4 months postnatally.
Psychological interventions focusing on interpersonal issues during pregnancy have also shown some promise (Spinelli & Endicott, Reference Spinelli and Endicott2003; Zlotnick et al. Reference Zlotnick, Miller, Pearlstein, Howard and Sweeney2006). This result contrasts with two previous ineffective prevention trials conducted in the same population by the present authors (T.S.B., C.J.M.) that employed external (research) therapists (Brugha et al. Reference Brugha, Wheatley, Taub, Culverwell, Friedman, Kirwan, Jones and Shapiro2000; Morrell et al. Reference Morrell, Spiby, Stewart, Walters and Morgan2000). Thus, individually delivered psychosocial interventions by professionals may be more effective postnatally (Dennis, Reference Dennis2005) when integrated into routine visits. For example, one other randomized trial of enhanced and personalized midwife-managed care in the early postnatal period also reported better EPDS outcomes at 7 weeks postnatally (Shields et al. Reference Shields, Reid, Cheyne, Holmes, McGinley, Turnbull and Smith1997). The similarity of both sets of research findings runs counter to the suggestion that our result is a chance, fortuitous finding.
Study limitations need to be considered given the potential public health significance of the results. A potential limitation of the study is that the EPDS subgroup by IG interaction tests have limited power to detect anything other than large interactions (Montgomery et al. Reference Montgomery, Peters and Little2003). The CI for the OR for the interaction effect at 6 months after adjustment for covariates (0.44–1.87) was relatively wide, so we cannot exclude such an effect, although the point estimate of the OR of 0.91 suggests this is unlikely. However, the interaction was not of primary interest and the trial was adequately powered to detect a reasonable target intervention effect, equivalent to an OR of 0.63. A full clinical interview was not performed on all CAU and IG EPDS-negative women and our primary outcome was thus not clinically assessed depression caseness. However, the EPDS is a well-established, reliable indicator of likelihood of clinical depression (Gaynes et al. Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2005). Bias may arise with cluster randomization because individual recruitment occurs after randomization (Puffer et al. Reference Puffer, Torgerson and Watson2005). According to Table 1, the profiles of IG and CAU women were remarkably balanced but, nevertheless, our analysis adjusted for key predictors of depression outcome. A planned replication and extended generalization of findings will be needed in other populations.
Rose (Reference Rose1992) argued that more people would benefit from a universal intervention. In our treatment trial (Morrell et al. Reference Morrell, Warner, Slade, Dixon, Walters, Paley and Brugha2009b) we found that 31 (11.4%) of 271 EPDS positive women benefited from intervention. In this prevention analysis, in EPDS-negative women, 46 (3.1%) of 1474 women benefited. Accordingly, the new intervention policy benefited far more women in the prevention (EPDS-negative) than in the treatment (EPDS positive) groups. Our finding that mental health benefits did not diminish over 18 months of follow-up contrasts with the short-term effectiveness of depression interventions in general and may reflect the ongoing extended contact that HVs often have with postnatal women. Additional analyses from this trial showed the probability that the intervention is cost-effective at various ‘threshold values’ of a quality-adjusted life year (QALY). In the range of QALY values between £20 000 and £30 000, the probability of the intervention being cost-effective is over 99% (Brugha et al. Reference Brugha, Morrell, Slade and Dixon2009). Thus, costs were lower and outcomes better in IG compared to CAU women, a concept often referred to by economists as ‘dominance’.
Until now, governments and policy makers have held back from investing significantly in depression prevention programs, arguably because of lack of convincing evidence for policy change and cost. To date, there have been no large-scale trials testing whether universal prevention effects have occurred across a whole population. There is now new evidence for clinically significant, useful and persistent reductions in the prevalence of depression in a key part of the population, women following childbirth, which requires further independent evaluation.
Acknowledgments
The trial was commissioned, funded and sponsored by the National Health Service (NHS), England, R&D Health Technology Assessment (HTA) Programme. [Registered in Current Controlled Trials as an International Standard Randomized Controlled Trial, number ISRCTN92195776.]
Declaration of Interest
None.








