Transcranial direct current stimulation (tDCS) is a novel non-pharmacological intervention consisting of administering direct, weak electric currents into the brain using electrodes placed on the scalp; these currents are able to induce neuromodulatory changes in cortical activity beyond the period of stimulation. Reference Brunoni, Nitsche, Bolognini, Bikson, Wagner and Merabet1 tDCS has been intensively investigated for the treatment of neuropsychiatric disorders in the past 15 years, Reference Brunoni, Nitsche, Bolognini, Bikson, Wagner and Merabet1 particularly major depression. Its antidepressant effects are premised on findings of relative left dorsolateral prefrontal cortex (DLPFC) underactivity (i.e. prefrontal dysfunction as indicated by reduced regional blood flow or glucose metabolism of the left DLPFC) that could be restored through anodal stimulation of this area, thus ameliorating depressive symptoms. Reference Brunoni, Ferrucci, Fregni, Boggio and Priori2 Randomised, sham-controlled clinical trials (RCTs) conducted hitherto have presented mixed results regarding its efficacy. Although recent meta-analyses suggest some efficacy when measuring depression symptoms using a continuous outcome Reference Kalu, Sexton, Loo and Ebmeier3–Reference Shiozawa, Fregni, Bensenor, Lotufo, Berlim and Daskalakis5 these meta-analyses were limited in their results as they used an aggregate data approach. Reference Simmonds, Higgins, Stewart, Tierney, Clarke and Thompson6 We aimed therefore to perform an individual patient data (IPD) meta-analysis. In contrast to aggregate data meta-analysis, an IPD approach uses the raw data of each participant collected from each study. IPD is more accurate in estimating the efficacy of an intervention as aggregate data meta-analyses present only summary estimates of efficacy. IPD meta-analysis is also superior to the aggregate data approach for obtaining predictors of treatment outcome, as the characteristics of each patient are assessed instead of the mean and frequency values obtained in the traditional aggregate data meta-analysis. Our objectives were twofold: (a) to provide precise estimates of tDCS efficacy based on continuous (depression improvement) and categorical (response and remission rates) outcomes and (b) to identify variables associated with tDCS efficacy.
Method
Systematic review
We first performed a systematic review according to the recommendations of the Cochrane Collaborative group and PRISMA guidelines. Reference Liberati, Altman, Tetzlaff, Mulrow, Gotzsche and Ioannidis7 The first two authors independently searched the literature and screened the search results. Differences were resolved by consensus. We also contacted experts in the field for unpublished articles and checked reference lists from recent systematic reviews. Our search was performed using MEDLINE, EMBASE, Web of Knowledge and Scopus databases (for the search strategy, please check the online supplement). We searched from the first date available until 1 January 2015. We only included randomised, sham-controlled trials with at least ten patients per arm (i.e. initial tDCS studies were not included) in which IPD were available. Case reports, series of cases, non-controlled trials, trial protocols, systematic reviews and meta-analyses were excluded.
Quality assessment
Methodological quality of each trial was assessed using two recognised checklists: (a) the Cochrane risk of bias tool Reference Higgins and Green8 that evaluates studies on the basis of selection, performance, detection, attrition and reporting biases and; (b) the PEDro scale, in which ten criteria are used for internal validity. Reference de Morton9 The PEDro cut-offs are 9–10 (excellent); 6–8 (good); 4–5 (fair) and below 4 (poor). Reference de Morton9 If the information was unclear, the first and/or the last authors of each RCT were contacted to request additional data.
Outcome measures
Outcome measures were:
-
(a) clinical response, defined as ⩾50% improvement from baseline to end-point depression scores;
-
(b) remission, defined as Montgomery–Åsberg Depression Rating Scale (MADRS) ⩽10, Hamilton Rating Scale for Depression (HRSD)-17 ⩽7, HRSD-21 ⩽8 and HRSD-24 ⩽9, according to standardised criteria, Reference Keller10–Reference Zimmerman, Posternak and Chelminski12 at end-point. This approach was used because even studies using the same scale differed regarding the remission cut-off point;
-
(c) depression improvement, estimated as the difference in z-scores (that was used for standardising depression symptoms and improvement across the studies, which used different scales) from baseline to end-point;
-
(d) acceptability (number of participants who dropped out in the active and sham groups at end-point).
Whether a study used more than one depression scale, we used the scale correspondent to the primary outcome. Moreover, when a study did not specify whether the MADRS or the HRSD was used as the primary outcome scale, we defined a priori that the HRSD scale would be used to calculate the outcome measures (response, remission and effect size). In fact, we could not use the same scale for our analyses because no single scale was unanimously used in all studies. For crossover (within-participants) RCTs, we considered only the data from the parallel (between-participants) phase. Finally, analyses were performed in the intention-to-treat samples, using the last observation carried forward for imputing missing data, which were considered to be missed at random.
Predictor variables – multivariate analysis
As one of the objectives of this trial, we assessed potential predictor (independent) variables. The main dependent variable was clinical response since it is more straightforward to interpret than depression improvement. Compared with remission, there were more patients presenting clinical response (hence, greater statistical power) and also remission presented heterogeneity of cut-offs. However, we also analysed depression improvement and remission separately. The collected predictor variables are described in the online supplement.
Data analysis
Baseline variables were described using mean and standard deviation for continuous variables and rates for categorical variables. Accordingly, values across groups were compared using one-way ANOVAs or the χ2 test respectively. For the outcome measures we performed an IPD, aggregate data (‘one-stage’) meta-analysis. Although both ‘one-stage’ and ‘two-stage’ IPD meta-analyses are commonly used, we chose the one-stage analysis as being more suitable for exploring predictors of response (for reviews, see Simmonds et al Reference Simmonds, Higgins, Stewart, Tierney, Clarke and Thompson6 and Debray et al Reference Debray, Moons, Abo-Zaid, Koffijberg and Riley13 ). The effect size was measured in terms of odds ratios (ORs) for response and remission and difference in z-scores for assessing depression improvement. The z-score was used for standardising depression symptoms and improvement across the studies, which used different scales. The z-score of each participant was obtained at study baseline and end-point by subtracting the group mean from the individual depression score and then dividing by the group standard deviation. Finally, we estimated the number needed to treat (NNT) based on the odds ratios for response and remission. NNT is a measurement used to assess and compare the effectiveness of clinical interventions; the higher the NNT, the less effective one intervention is. In our study, NNT represents the number of patients with clinical depression it is necessary to treat (i.e. to receive active tDCS) for one additional patient to experience response or remission. NNT provides a value that is relative to the control.
Analyses were performed in Stata 12 using the commands xtmixed, xtmelogit and ipdforest, according to the recommendations of a recent guideline for performing IPD one-stage meta-analysis in Stata. Reference Kontopantelis and Reeves14 The variance–covariance structure was considered to be independent; this structure allows a distinct variance for each random effect within a random-effects equation and assumes all covariances to be zero. These analyses were used to estimate the effect size of active v. sham tDCS and to graph the forest plots.
For categorical and continuous outcomes, mixed-effects logistic and linear regression models were respectively used with ‘study’ as a random-effects variable and ‘tDCS’ (active/sham) nested in the variable ‘study’. ‘Study’ contained six categories that are not ordinal and therefore were dummy coded to be included in the model. The study of Brunoni et al Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15 was the reference group for this model since it contained the largest number of patients and provided the best power for comparisons. For the logistic regression of response, we had to collapse the studies in order to avoid non-convergence as the number of events was low – therefore the studies of Palm et al Reference Palm, Schiller, Fintescu, Obermeier, Keeser and Reisinger16 and Blumberger et al Reference Blumberger, Tran, Fitzgerald, Hoy and Daskalakis17 were collapsed into one meta-study. For the same reason, for the logistic regression of remission the studies of Palm et al, Reference Palm, Schiller, Fintescu, Obermeier, Keeser and Reisinger16 Blumberger et al Reference Blumberger, Tran, Fitzgerald, Hoy and Daskalakis17 and Bennabi et al Reference Bennabi, Nicolier, Monnin, Tio, Pazart and Vandel18 were collapsed into one meta-study.
To identify predictors of improvement, response and remission, we ran several univariate analyses, initially using only one predictor variable at a time, although forcing the variable ‘study’ into all models because it is potentially an important confounder, even though it may not necessarily be a significant predictor of response. The predictor analyses were only performed in patients receiving active tDCS (i.e. excluding the sham group). Predictors that were measured in all studies and presented a P<0.1 were further included in the multivariate analyses, in which initially all significant (P<0.1) predictors were imputed and then successively removed if they were not significant at a two-tailed P threshold of 0.05 (stepwise backward method); best fits were chosen based on the Wald statistics. Finally, to assess acceptability, we compared drop-out rates between active and sham tDCS using the random-effects odds ratio.
Results
Overview
Our MEDLINE search initially retrieved 116 potentially eligible studies. Of those, 107 were excluded based on their title and abstract and the other two after a full study assessment, as they did not match the eligibility criteria. Also, one reference was excluded because individual data were not available. Finally, six RCTs were included in our analysis. Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15–Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20 We did not identify any studies in the other databases that were not also found in the MEDLINE search (see online supplement).
Study description and quality assessment
Table 1 describes the main eligibility criteria of the included studies; the risk of bias is shown in the online supplement. All trials were considered to be of low bias risk according to the Cochrane risk of bias tool and achieved maximum scores on the PEDro scale.
Table 1 Summary of the included studies a

| Palm et al (2012) Reference Palm, Schiller, Fintescu, Obermeier, Keeser and Reisinger16 | Brunoni et al (2013) Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15 | Loo et al (2012) Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20 | Loo et al (2010) Reference Loo, Sachdev, Martin, Pigot, Alonzo and Malhi19 | Blumberger et al (2012) Reference Blumberger, Tran, Fitzgerald, Hoy and Daskalakis17 | Bennabi et al (2015) Reference Bennabi, Nicolier, Monnin, Tio, Pazart and Vandel18 | |
|---|---|---|---|---|---|---|
| Design | RCT, crossover | 2-arm RCT (tDCS, drug) | RCT | RCT | RCT | RCT |
| Clinicaltrials.gov (NCT) | 00667680 | 01033084 | 00763230 | 00256438 | 01078948 | 01428804 |
| Start date | Mar 2007 | Mar 2010 | Sep 2008 | Jan 2006 | Feb 2008 | N/A |
| End date | Nov 2008 | Feb 2011 | Feb 2011 | Dec 2008 | Oct 2010 | N/A |
| Main inclusion criteria | MDD | MDD, low suicide risk, antidepressant-free |
MDD | MDD, ⩾3 years | Non-psychotic MDD | Refractory MDD |
| Depression cut-off | HRSD-24 ⩾18 | HRSD-17 ⩾17 | MADRS ⩾20 | MADRS ⩾20 | HRSD-17 ⩾21 | MADRS ⩾25 |
| Bipolar disorder | Allowed | Excluded | Allowed | Excluded | Excluded | Excluded |
| Main exclusion criteria | Other Axis I disorders, suicidality, neurological disorders |
Other Axis I disorders, any Axis II disorders, neurological disorders |
Other Axis I disorders, failure of ECT, neurological disorders |
Other Axis I disorders, failure of ECT, neurological disorders |
Other Axis I, any Axis II, neurological disorders, use of ACV |
Neurological disorders, use of FGA, failure of ECT |
| Age range, years | 36–79 | 18–65 | 23–78 | 19–65 | 24–62 | 30–81 |
| Primary outcome measure | HRSD-24 | MADRS | MADRS | MADRS | HRSD-17 | HRSD-21 |
| Intention-to-treat analysis | Yes | Yes | Yes | Yes | Yes | Yes |
| Sample size, n | 22 | 120 | 60 | 40 | 24 | 23 |
| Stimulation characteristics | ||||||
| Device | Eldith DC | Chattanooga Ionto device | Eldith DC | Eldith DC | CX-6650 | Eldith DC |
| Masking | Double-blind | Double single-blinded | Double-blind | Double-blind | Double-blind | Double-blind |
| Anode | F3 | F3 | pF3 | F3 | F3 | F3 |
| Cathode | RSO | F4 | F8 | RSO | F4 | RSO |
| Number of sessions/frequency | 10/once a day | 12/once a day | 15/once a day | 5/every other day | 15/once a day | 10/twice a day |
| Weeks of stimulation | 2 | 2 (+ 2 sessions every other week) |
3 | 2 | 3 | 1 |
| Current density, A/m2 | 0.29/0.57 | 0.80 | 0.57 | 0.29 | 0.57 | 0.57 |
| Current, mA | 1–2 | 2 | 2 | 1 | 2 | 2 |
| Session duration, min | 20 | 30 | 20 | 20 | 20 | 30 |
| Total charge, C | 12–24 | 43.2 | 36 | 6 | 36 | 36 |
| Total charge density, C/m2 | 3248/6857 | 17280 | 10285 | 1714 | 10285 | 10285 |
RCT, randomised clinical trial; tDCS, transcranial direct current stimulation; MDD, major depressive disorder; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scales; ECT, electroconvulsive therapy; ACV, anticonvulsant drugs; FGA, first-generation antipsychotic;
F3 or pF3, left dorsol ateral prefrontal cortex; F4, right dorsolateral prefrontal cortex; F8/RSO, right supraorbital area.
a. For all studies, sham method consisted of a brief (5–60s), initial period of active stimulation, followed by no stimulation during the rest of the session.
Main outcomes
Data from 289 patients were analysed. Their distribution across the six studies was significantly different for almost all baseline clinical and demographic variables (Table 2). The between-study heterogeneity was addressed as the variable ‘study’ was forced into all models.
Table 2 Clinical and demographic characteristics of the included studies at baseline

| All patients (n = 289) |
Palm et al (2012)
Reference Palm, Schiller, Fintescu, Obermeier, Keeser and Reisinger16
(n = 22) |
Brunoni et al (2013)
Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15
(n = 120) |
Loo et al (2012)
Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20
(n = 60) |
Loo et al (2010)
Reference Loo, Sachdev, Martin, Pigot, Alonzo and Malhi19
(n = 40) |
Blumberger et al
(2012) Reference Blumberger, Tran, Fitzgerald, Hoy and Daskalakis17 (n = 24) |
Bennabi et al (2015)
Reference Bennabi, Nicolier, Monnin, Tio, Pazart and Vandel18
(n = 23) |
|
|---|---|---|---|---|---|---|---|
| Women, n (%) a | 180 (62.3) | 14 (63.6) | 82 (68.3) | 28 (46.7) | 22 (55.0) | 20 (83.3) | 14 (60.9) |
| Age, years: mean (s.d.) a | 47.2 (13.4) | 57.1 (11.6) | 42.3 (12.7) | 48.2 (12.4) | 47.3 (11.3) | 47.3 (10.7) | 60.8 (13.3) |
| Characteristics of depression | |||||||
| Age at onset, years: mean (s.d.) a | 31.6 (14) | 42.2 (12.4) | 30.6 (13.2) | 28.3 (12.4) | 31.5 (14.1) | 21.5 (12) | 42.1 (13.5) |
| Bipolar disorder, n (%) a | 11 (3.8) | 2 (9.1) | 0 | 8 (13.3) | 0 | 0 | 1 (4.3) |
| Melancholic, n (%) | 100 (34.6) | 4 (18.2) | 31 (25.8) | 31 (51.7) | 17 (42.5) | 2 (8.3) | 15 (65.2) |
| Atypical, n (%) a | 81 (28.0) | 1 (4.5) | 58 (48.3) | N/A | N/A | 2 (8.3) | 2 (8.7) |
| Treatment-resistant depression, n (%) a | 149 (51.6) | 21 (95.5) | 45 (37.5) | 24 (40) | 13 (32.5) | 100 | 23 (100.0) |
| Failed trials, mean (s.d.) a | 2.3 (2.4) | 3.6 (1.8) | 1.8 (2.3) | 1.7 (1.9) | 1.5 (1.9) | 4.2 (2.3) | 5.1 (1.8) |
| Past episodes, mean (s.d.) | 5.5 (7.8) | 7.9 (6.3) | 5.3 (8.8) | N/A | N/A | 5.4 (7.3) | 4.2 (2.4) |
| Duration of current depressive episode, weeks: mean (s.d.) a |
23.4 (36.9) | 8.3 (12.3) | 15.8 (18) | 44.3 (61.1) | 18.7 (16.5) | 46.8 (54.1) | 9.9 (6.2) |
| Anxiety disorder, n (%) a | 123 (42.6) | 5 (22.7) | 70 (58.3) | 9 (15.0) | 21 (52.5) | 5 (20.8) | 13 (56.5) |
| Severe depression, n (%) | 160 (55.4) | 21 (95.5) | 68 (56.7) | 30 (50.0) | 17 (42.5) | 13 (54.2) | 11 (47.8) |
| Treatments of the current depressive episode | |||||||
| Psychotherapy, n (%) a | 86 (29.8) | 22 (100) | 12 (10.0) | N/A | N/A | N/A | 15 (65.2) |
| Electroconvulsive therapy use, n (%) a | 12 (4.1) | 6 (27.3) | 2 (1.7) | 0 | 0 | 4 (16.7) | 0 |
| Repetitive transcranial magnetic stimulation use, n (%) a |
5(1.7) | 0 | 1 (0.8) | 0 | 0 | 0 | 4 (17.4) |
| Tricyclic antidepressants, n (%) a | 27 (9.3) | 13 (59.1) | 0 | 7 (11.7) | 4 (10.0) | 3 (12.5) | 0 |
| Selective serotonin reuptake inhibitors, n (%) a | 66 (22.8) | 16 (72.7) | 0 | 12 (20.0) | 11 (27.5) | 4 (16.7) | 23 (100) |
| Serotonin-noradrenaline reuptake inhibitors, n (%) a | 35 (12.1) | 2 (9.1) | 0 | 19 (31.7) | 5 (12.5) | 9 (37.5) | 0 |
| Anticonvulsants, n (%) a | 8 (2.8) | 3 (13.6) | 1 (0.8) | 0 | 0 | 0 | 4 (17.4) |
| Antipsychotics, n (%) a | 47 (16.3) | 18 (81.8) | 0 | 11 (18.3) | 6 (15.0) | 1 (4.2) | 11 (47.8) |
| Benzodiazepines, n (%) a | 56 (19.4) | 10 (45.5) | 23 (19.2) | 1 (1.7) | 0 | 8 (33.3) | 14 (60.9) |
N/A not available.
a. Variables in which groups were significantly different at baseline (P<0.01).
Active tDCS was superior to sham for response (34% v. 19% respectively; OR = 2.44, 95% CI 1.38–4.32, P = 0.002), remission (23.1% v. 12.7% respectively, OR = 2.38, 95% CI 1.22–4.64, P = 0.002) and depression improvement (β = 0.347, 95% CI 0.12–0.57, P = 0.002). The NNTs for response and remission were, respectively, 7 (95% CI 4.04–20.7) and 9 (95% CI 5.2–63). The I 2 of all models were ⩽2.5%, which are indicative of low heterogeneity (Fig. 1).

Fig. 1 Individual patient data meta-analysis comparing active v. sham transcranial direct current stimulation (tDCS) in terms of (a) response; (b) remission and (c) depression improvement.
Forest plot graphically illustrating the comparative efficacy of active with sham tDCS. For each study the relative strength of treatment effects is represented, the square represents the relative weight of each study, the vertical bar is the 95% confidence interval. The rhombus represents the overall effect. Values >1 and >0 v. <1 and <0 represent positive and negative effects of active v. sham tDCS for categorical and continuous outcomes respectively.
Predictors of clinical response
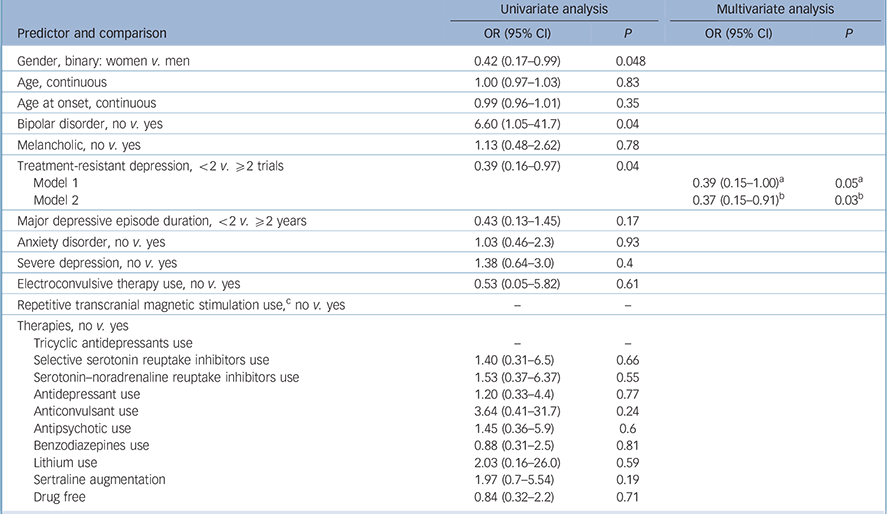
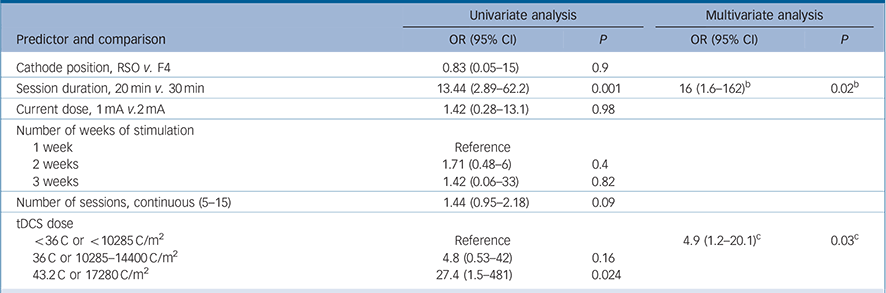
The variables ‘use of psychotherapy’, ‘number of past episodes’ and ‘atypical depression’ were not included in the predictor models because not all studies collected these data. Also, we could not explore the interaction between number of weeks of stimulation and frequency of sessions because of collinearity. The following predictors achieved the significance threshold in the univariate analyses and were included in the multivariate analyses: female gender, bipolar disorder, treatment-resistant depression and the tDCS characteristics session duration, number of sessions and tDCS dose (Tables 3 and 4).
Table 3 Clinical and demographic variables: univariate and multivariate analyses of predictors of response to transcranial direct current stimulation (tDCS)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Predictor and comparison | OR (95% CI) | P | OR (95% CI) | P |
| Gender, binary: women v. men | 0.42 (0.17–0.99) | 0.048 | ||
| Age, continuous | 1.00 (0.97–1.03) | 0.83 | ||
| Age at onset, continuous | 0.99 (0.96–1.01) | 0.35 | ||
| Bipolar disorder, no v. yes | 6.60 (1.05–41.7) | 0.04 | ||
| Melancholic, no v. yes | 1.13 (0.48–2.62) | 0.78 | ||
| Treatment-resistant depression, <2 v. ⩾2 trials | 0.39 (0.16–0.97) | 0.04 | ||
| Model 1 | 0.39 (0.15–1.00) a | 0.05 a | ||
| Model 2 | 0.37 (0.15–0.91) b | 0.03 b | ||
| Major depressive episode duration, <2 v. ⩾2 years | 0.43 (0.13–1.45) | 0.17 | ||
| Anxiety disorder, no v. yes | 1.03 (0.46–2.3) | 0.93 | ||
| Severe depression, no v. yes | 1.38 (0.64–3.0) | 0.4 | ||
| Electroconvulsive therapy use, no v. yes | 0.53 (0.05–5.82) | 0.61 | ||
| Repetitive transcranial magnetic stimulation use, c no v. yes | – | – | ||
| Therapies, no v. yes | ||||
| Tricyclic antidepressants use | – | – | ||
| Selective serotonin reuptake inhibitors use | 1.40 (0.31–6.5) | 0.66 | ||
| Serotonin-noradrenaline reuptake inhibitors use | 1.53 (0.37–6.37) | 0.55 | ||
| Antidepressant use | 1.20 (0.33–4.4) | 0.77 | ||
| Anticonvulsant use | 3.64 (0.41–31.7) | 0.24 | ||
| Antipsychotic use | 1.45 (0.36–5.9) | 0.6 | ||
| Benzodiazepines use | 0.88 (0.31–2.5) | 0.81 | ||
| Lithium use | 2.03 (0.16–26.0) | 0.59 | ||
| Sertraline augmentation | 1.97 (0.7–5.54) | 0.19 | ||
| Drug free | 0.84 (0.32–2.2) | 0.71 | ||
a. Results are from Model 1 that includes treatment-resistant depression and session duration.
b. Results are for Model 2 that includes treatment-resistant depression and tDCS dose.
c. We were not able to analyse repetitive transcranial magnetic stimulation (rTMS) use as a predictor due to the low number of patients that received rTMS.
Table 4 Transcranial direct current stimulation (tDCS) characteristics: univariate and multivariate analyses of predictors of response to tDCS a
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Predictor and comparison | OR (95% CI) | P | OR (95% CI) | P |
| Cathode position, RSO v. F4 | 0.83 (0.05–15) | 0.9 | ||
| Session duration, 20min v. 30 min | 13.44 (2.89–62.2) | 0.001 | 16 (1.6–162) b | 0.02 b |
| Current dose, 1 mA v.2mA | 1.42 (0.28–13.1) | 0.98 | ||
| Number of weeks of stimulation | ||||
| 1 week | Reference | |||
| 2 weeks | 1.71 (0.48–6) | 0.4 | ||
| 3 weeks | 1.42 (0.06–33) | 0.82 | ||
| Number of sessions, continuous (5–15) | 1.44 (0.95–2.18) | 0.09 | ||
| tDCS dose | ||||
| <36C or < 10285 C/m2 | Reference | 4.9 (1.2–20.1) c | 0.03c | |
| 36 C or 10285–14400 C/m2 | 4.8 (0.53–42) | 0.16 | ||
| 43.2 C or 17280 C/m2 | 27.4 (1.5–481) | 0.024 | ||
RSO, right supraorbital area; F4, right dorsolateral prefrontal cortex.
a. Number of sessions and number of weeks of stimulation are different variables because there were studies performing tDCS sessions once daily, twice daily or on alternated days. Please refer to the main text for details.
b. Results are from Model 1 that includes treatment-resistant depression and session duration.
c. Results are for Model 2 that includes treatment-resistant depression and tDCS dose.
In the multivariate analyses, the first models included the abovementioned predictors – although, for tDCS, each variable was imputed in a different model since they are redundant. In these first analyses, number of sessions was not significant, although session duration and tDCS dose were. Also, gender was not a significant predictor in both models and the variable bipolar disorder was excluded because of collinearity (caused by the low number of cases). Therefore, the final multivariate model for response included the variables treatment-resistant depression (Table 3) and tDCS dose/session duration (Table 4).
Predictors of remission and depression improvement
The only significant univariate predictors of remission were treatment-resistant depression, melancholia, session duration and tDCS dose (online supplement). It was not possible to identify a multivariate model for remission, since non-convergence was detected in most models, as the number of events was still low even after collapsing the studies.
For depression improvement, severe depression, treatment-resistant depression, bipolar depression, female gender, use of antidepressant drugs, sertraline augmentation, number of sessions and tDCS dose were significant univariate predictors in the linear model (online supplement). For the multivariate analyses, number of sessions/tDCS dose were analysed separately. Also, sertraline augmentation/use of antidepressant drugs were analysed separately as they presented complete separation (i.e. in the study in which sertraline was augmented to tDCS, patients were antidepressant free; whereas there was no drug augmentation in studies in which patients were using antidepressant drugs). Therefore, the first four models included initially all significant predictors. However, models including use of antidepressant drugs were further excluded since this variable did not achieve significance (P's>0.27). Also, gender was further excluded in the remaining models because it did not achieve significance (P's>0.08). Therefore, the final models included bipolar depression, treatment-resistant depression, severe depression, sertraline augmentation and number of sessions/tDCS dose (online supplement).
Acceptability
The drop-out rates between active (10.1%) and sham (12.2%) groups were not significantly different (OR = 0.78, 95% CI 0.33–1.82; P = 0.57) (online supplement).
Discussion
Main findings and comparison with findings for other treatments
In this first IPD meta-analysis of tDCS as a treatment for acute depressive episodes, we collected data from 289 patients (147 and 142 in the active and sham groups respectively) from six RCTs. We found significant effect sizes of efficacy of active v. sham tDCS in terms of depression improvement, response and remission, with overall response and remission rates of active tDCS of 34% and 23% respectively and NNTs of 7 and 9. Our work has some strengths when compared with findings from aggregate data meta-analyses. For instance, using an IPD meta-analytic approach, we were able to provide more precise estimates of effect size than previous aggregate data meta-analyses. This approach also allowed us to use the same operational definition of response and remission for all studies, in contrast to aggregate data meta-analyses that used the definition provided by the study, limiting their generalisability. tDCS was also an acceptable treatment, as the drop-out rates between active and sham groups were not statistically different.
The NNT for response was similar to a Cochrane meta-analysis assessing the efficacy of antidepressant drug treatments in primary care, Reference Arroll, Elley, Fishman, Goodyear-Smith, Kenealy and Blashki21 which observed NNTs for response of 9 and 7 for TCAs and selective serotonin reuptake inhibitors (SSRIs), respectively. Another meta-analysis Reference Undurraga and Baldessarini22 reviewed 122 antidepressant drug trials published in the past 30 years and observed an NNT of 8 for antidepressant drug treatment in depression. Our findings are also in the same range as a recent active v. sham repetitive transcranial magnetic stimulation (rTMS) meta-analysis for depression (n = 1371) that found response and remission NNTs of 6 and 8 respectively. Reference Berlim, van den Eynde, Tovar-Perdomo and Daskalakis23 In that study, the absolute rates of response and remission in the active group of rTMS (29.3% and 18.6% respectively) were similar to the rates observed in our study on tDCS. The absolute rate of tDCS response was also similar to that observed in the STAR*D trial, a multicentric, pragmatic antidepressant treatment study that enrolled up to 3000 patients. Reference Rush, Trivedi, Wisniewski, Stewart, Nierenberg and Thase24 Our findings indicates two potential applications for tDCS in the therapeutic arsenal for depression: in primary care settings and as a non-pharmacological, neuromodulatory therapy for depression. Another aspect corroborating the role of tDCS in primary care is its characteristics of low-cost, ease of use, portability and a benign profile of side-effects. Reference Brunoni, Nitsche, Bolognini, Bikson, Wagner and Merabet1 Given these results we discuss below potential factors that can influence response such as patient selection (best responders) and parameters of stimulation based on our multivariate regression models results.
Factors influencing response to tDCS
Treatment-resistant depression was unanimously associated with lower tDCS efficacy in all predictor models. This finding had already been found in a larger tDCS trial and its follow-up study, Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15,Reference Valiengo, Bensenor, Goulart, de Oliveira, Zanao and Boggio25 although not detected in previous aggregate data meta-regressions, possibly because of lack of power. This finding is in line with outcomes observed for pharmacotherapy, Reference Trivedi, Fava, Wisniewski, Thase, Quitkin and Warden26 rTMS Reference Fregni, Marcolin, Myczkowski, Amiaz, Hasey and Rumi27,Reference Lisanby, Husain, Rosenquist, Maixner, Gutierrez and Krystal28 and electroconvulsive therapy, Reference Sackeim, Prudic, Devanand, Nobler, Lisanby and Peyser29 and further ratifies the notion that the optimal use of tDCS would be in primary care and other low-refractory sample settings. Conversely, tDCS protocols targeting refractory samples should be optimised, possibly using higher tDCS doses as its efficacy is lower in such samples.
tDCS dose constituted an important and independent predictor of better efficacy and it was also associated with greater improvement in active v. sham groups. The association of higher tDCS doses with treatment efficacy is in keeping with prior clinical trials in the field of non-invasive brain stimulation. For rTMS, for instance, the first studies employed a small number (5–10 days) of sessions, whereas pivotal studies performed 3–6 weeks of sessions. Reference George, Lisanby, Avery, McDonald, Durkalski and Pavlicova30 A recent review Reference Lefaucheur, Andre-Obadia, Antal, Ayache, Baeken and Benninger31 indicated an association between treatment efficacy of rTMS and more intensive protocols (for example days of stimulation and number of pulses). Nonetheless, previous aggregate data meta-analyses indicated either a lack of association Reference Berlim, Van den Eynde and Daskalakis4 or a non-significant trend for a positive association between tDCS ‘dose’ and efficacy, Reference Shiozawa, Fregni, Bensenor, Lotufo, Berlim and Daskalakis5 probably because this type of analysis is underpowered, compared with IPD meta-analysis, in identifying predictors of outcome.Reference Simmonds, Higgins, Stewart, Tierney, Clarke and Thompson6
Importantly, there is no consensus regarding how to measure ‘tDCS dose’ in RCTs. We therefore employed different approaches, considering session duration, current dose and number of sessions. When using charge density, a measurement that takes into account all these variables (current, duration and number of sessions), we estimated a tDCS dose that was an independent predictor for both continuous and categorical multivariate analyses. This finding is relevant for clinical application protocols as it indicates that there is an important dose-dependent effect for tDCS. On the other hand, we were not able to investigate the influence of electrode size (and thus the importance of current delivered per area) as only one study used 25 cm Reference Brunoni, Ferrucci, Fregni, Boggio and Priori2 (v. 35 cm2) electrodes. In addition, we could not identify whether current dose or number of sessions were more important for determining depression improvement, as neither was significantly associated with the outcome, although a longer session duration (30 v. 20 min) was positively associated with clinical response. From a clinical perspective, however, it is possible that increasing the number of sessions is more feasible than increasing current dose and session duration, which might become uncomfortable and tiresome, respectively, for patients. In this context, there should be further investigation of the optimal frequency interval between tDCS sessions – a variable that may be critical in inducing optimal neuroplasticity, Reference Brunoni, Nitsche, Bolognini, Bikson, Wagner and Merabet1 and if missing treatment sessions can be replaced at the end of the protocol without harming clinical efficacy, as previously suggested. Reference Zanao, Moffa, Shiozawa, Lotufo, Bensenor and Brunoni32
We found no interaction between concomitant pharmacotherapy and tDCS efficacy, in comparison with neurophysiological studies (for a review, see Stagg & Nitsche Reference Stagg and Nitsche33 ) that showed influence of pharmacotherapy on tDCS effects. However, these studies tested a single drug dose timed with the administration of a single tDCS session in healthy volunteers and measured a surrogate outcome (motor cortical excitability), whereas the RCTs included in our analyses performed tDCS sessions over several days over the prefrontal cortex in participants with depression and with chronic use of pharmacotherapy. Therefore the neurophysiological findings described may not necessarily reflect what is taking place in our sample. Also, although antidepressant drug use was a significant univariate predictor of depression improvement in a previous naturalistic finding in 82 patients with depression, Reference Brunoni, Ferrucci, Bortolomasi, Scelzo, Boggio and Fregni34 this study was less controlled than the present RCTs and measured outcomes immediately after 5 days of stimulation. Nonetheless, sertraline augmentation was an independent predictor of depression improvement, reflecting the results of Brunoni et al Reference Debray, Moons, Abo-Zaid, Koffijberg and Riley13 that showed that tDCS and sertraline simultaneously combined led to faster, greater depression improvement when compared with groups receiving only sertraline or only tDCS.
Bipolar depression was a univariate predictor of response and a multivariate predictor of depression improvement; however, the overall number of patients with bipolar disorder was low (3.8%) and, in fact, half of the RCTs did not include people with bipolar disorder, limiting the conclusions on tDCS efficacy. This highlights the urgent need for tDCS studies in bipolar disorder. Gender was also a univariate predictor of response. This finding can be explained by reasons such as: (a) all the RCTs used convenience samples – the recruited women could have presented with milder forms of depression than men – as usually women are more prone to spontaneously seek for medical care; (b) different antidepressant effects of tDCS according to gender, as observed in a recent rTMS meta-analysis for depression. Reference Kedzior, Azorina and Reitz35 Importantly, gender was not a significant predictor after adjustment for other confounders, indicating that the role of gender in determining tDCS efficacy is less important compared with other variables such as depression refractoriness and tDCS dose. Finally, depression severity was an independent predictor of depression improvement. A previous antidepressant meta-analysis also observed that drugs are more effective in more severe depression. Reference Kirsch, Deacon, Huedo-Medina, Scoboria, Moore and Johnson36 Such greater effect can also be a statistical artefact associated with clinical trial design, as there is more room for improvement where baseline severity is high (i.e. regression to the mean).
Acceptability of treatment
We could not measure the frequency of individual adverse effects because this assessment varied among studies and different questionnaires were used. Hence, we used the frequency of drop-out as a proxy of tDCS acceptability, finding that active and sham groups presented similar drop-out rates. This indicates that tDCS is a tolerable and acceptable treatment for acute depression. Also, the collected adverse effects were mild and transient.
Follow-up studies
Our work focused on the efficacy of tDCS during an acute depressive episode. Two of the included RCTs Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15,Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20 assessed relapse rates in tDCS responders during a long-term follow-up of 6 months. Both follow-up studies Reference Valiengo, Bensenor, Goulart, de Oliveira, Zanao and Boggio25,Reference Martin, Alonzo, Ho, Player, Mitchell and Sachdev37 presented similar findings regarding: (a) relapse rates of approximately 50% at the end of the follow-up; (b) treatment-resistant depression was a predictor of relapse and; (c) the decrease in tDCS regimen at month 3 was associated with an increase in relapse rates. Thus, an association between tDCS efficacy with treatment-resistant depression and tDCS dose was also observed in the continuation studies. Also, overall effectiveness of tDCS was poor. Therefore, further maintenance studies could assess whether more intensive tDCS treatment regimens – both in the acute and in the maintenance phases – could be associated with lower relapse rates at follow-up.
Strengths and limitations
Quality assessment revealed that studies used generally similar and acceptable methods of randomisation and masking. Regarding the sham method, the studies that used non-automated tDCS devices either observed that patients were not able to guess between active and sham tDCS Reference Loo, Sachdev, Martin, Pigot, Alonzo and Malhi19,Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20 or found that the sham procedure was as effective as the gold-standard placebo-pill. Reference Brunoni, Schestatsky, Lotufo, Bensenor and Fregni38
We did not observe other types of bias in the included studies, although most of them included small sample sizes and were heterogeneous. This issue was addressed by including the variable ‘study’ as a random-effects variable.
Only results from the study by Brunoni et al Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15 were significant when each study was analysed individually (Fig. 1), which is in line with the results observed in the corresponding clinical trials, excluding the continuous outcome of Loo et al Reference Loo, Alonzo, Martin, Mitchell, Galvez and Sachdev20 – significant in the original study (this occurred as different continuous outcomes were used in the original and in the present study – this strategy was employed to obtain an individual standard score). These discrepant findings occurred primarily because of the insufficient sample size that yielded non-significant findings in the original and in the present trials – conversely, the larger study by Brunoni et al Reference Brunoni, Valiengo, Baccaro, Zanao, Oliveira and Goulart15 presented significant findings. This issue was at least partly addressed by the statistical approach of one-stage IPD meta-analysis that assesses the net effect size by pooling data from each participant. Nonetheless, this observation highlights the need for further, larger RCTs for tDCS in the treatment of acute depressive episodes. Another possibility is the different sample characteristics across these studies such as depression refractoriness and use of other medications that could have influenced the efficacy of tDCS. In this context, Nitsche et al Reference Nitsche, Kuo, Karrasch, Wachter, Liebetanz and Paulus39 found that the SSRI citalopram enhanced the effects of anodal tDCS on motor cortical excitability measures. Thus, differences in medication may contribute to variation of antidepressant efficacy and the pharmacotherapy–tDCS interaction needs to be further investigated in depression.
The studies also used distinct criteria for refractoriness, chronicity, response and remission; these issues were addressed by standardising the same operational criteria for all studies. Finally, IPD data from a potential included study Reference Boggio, Rigonatti, Ribeiro, Myczkowski, Nitsche and Pascual-Leone40 were not available. However, considering that its results were positive, our main results would not change; in fact, its inclusion would probably increase the observed effect size.
Implications
This first IPD meta-analysis expanded evidence that active tDCS is superior to sham tDCS in terms of depression improvement, clinical response and remission for an acute depressive episode. Our findings also suggest that tDCS presents similar efficacy to antidepressant drugs and rTMS, although the total number of studies is still small. Treatment-resistant depression and higher tDCS dosages were negatively and positively, respectively, associated with treatment response in univariate and multivariate models; upcoming trials can be thus optimised according to these parameters. Other variables, such as presence of bipolar disorder, severe depression, sertraline augmentation and female gender were also identified in some models and should be explored in further studies.
Funding
A.R.B. is supported by the following grants: 2013 NARSAD Young Investigator from the Brain & Behavior Research Foundation (Grant Number 20493), 2013 FAPESP Young Researcher from the São Paulo State Foundation (Grant Number 20911-5) and National Council for Scientific and Technological Development (CNPq, Grant Number 470904). A.R.B. also received equipment from the Soterix company. F.P. received grants from neuroConn GmbH, Ilmenau, Germany and Brainsway Inc, Jerusalem, Israel. C.L. has received research grant funding from the Australian National Health and Medical Council and the Stanley Medical Research Foundation, and equipment from the Soterix company. D.M.B. currently receives compensation from: the Centre for Addiction and Mental Health, Toronto, Ontario. D.M.B. also receives research support from the Canadian Institutes of Health Research and the US National Institute of Health (NIH). D.M.B. receives research support and in-kind equipment support from Brainsway Ltd for an investigator-initiated clinical trial and a sponsor-initiated clinical trial. D.M.B. receives in-kind equipment support from Tonika-Magventure for an investigator-initiated study. E.H. and D.B. received a grant from Lundbeck Foundation. Z.J.D. received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc. This work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the Brain and Behaviour Research Foundation and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.










eLetters
No eLetters have been published for this article.