Introduction
Individuals with obsessive-compulsive disorder (OCD) suffer from obsessions, which are recurrent intrusive thoughts, and/or compulsions, which are ritualistic repetitive behaviours or mental acts (APA, 1994). This debilitating condition has a lifetime prevalence of 2–3% (Robins et al. Reference Robins, Helzer, Weissman, Orvaschel, Gruenberg, Burke and Regier1984). The clinical presentation of OCD is heterogeneous, not only with large variations between individuals in the nature of their obsessions and compulsions as well as treatment response, but also with changes in symptoms and their severity over time within individuals (Miguel et al. Reference Miguel, Leckman, Rauch, do Rosario-Campos, Hounie, Mercadante, Chacon and Pauls2005). Brain abnormalities have been noted in OCD patients in the prefrontal cortex, including the orbitofrontal cortex, parietal cortex and striatum (Menzies et al. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008). Notwithstanding the variability in clinical presentation, consistent impairment in volitional suppression of simple actions has led to the suggestion that response inhibition deficits may provide a useful intermediate marker of brain dysfunction, or endophenotype, for OCD (Chamberlain et al. Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005). Such an endophenotype could facilitate greater clarity in discerning the diagnostic classification, aetiological understanding, as well as the course, outcome and treatment strategies for OCD.
Dysfunction of inhibitory control has long been theorized to be a central feature of OCD. Impairments in intentionally inhibiting simple motor actions have been clearly demonstrated in OCD patients using the stop signal task (Chamberlain et al. Reference Chamberlain, Fineberg, Blackwell, Robbins and Sahakian2006a). In this task, participants perform a speeded identification go task to simple stimuli (e.g. pressing right and left keys in response to right and left pointing arrows, respectively). On occasion a sudden auditory stop signal follows the go stimuli signalling to inhibit the planned pre-potent response (Logan & Cowan, Reference Logan and Cowan1984). Importantly, the deficit is specific to inhibition in this task, as OCD patients do not demonstrate additional impairment in their go response. Consistent with the proposal of impaired inhibition as an endophenotype for OCD, is the finding of impaired inhibition of simple motor responses in unaffected first-degree relatives of individuals with OCD (Chamberlain et al. Reference Chamberlain, Fineberg, Menzies, Blackwell, Bullmore, Robbins and Sahakian2007b). Recent evidence has further suggested that there are structural brain differences linked to inhibitory processing using the stop signal task, distinguishing both OCD patients and their unaffected first-degree relatives from healthy controls (Menzies et al. Reference Menzies, Achard, Chamberlain, Fineberg, Chen, del Campo, Sahakian, Robbins and Bullmore2007).
The present study examined whether an endophenotype of impaired stopping could be extended to inhibition of ongoing thoughts. To investigate whether individuals with OCD terminate their ongoing processing effectively when given a salient external switch signal, we used a novel adaptation of the thought stop-signal task (TSST) (Logan, Reference Logan1985). Participants performed speeded word judgements and, as in the standard stop-signal task, were occasionally signalled to stop. By examining performance on a subsequent presentation, we investigated whether the thoughts underlying the actions were also inhibited along with the response. Speeding from the repeated presentations was used to determine whether the original thoughts went on to completion when the overt responses were inhibited. The repetition effect (repetition priming) refers to the faster response found when the same judgement is made again. Previous studies have suggested that simple thoughts that go on to completion lead to greater repetition priming compared with inhibited thoughts (Logan, Reference Logan1985). While repetition priming has been linked to the left inferior frontal gyrus (Wagner et al. Reference Wagner, Koutstaal, Maril, Schacter and Buckner2000), response inhibition has been linked to a network involving the right inferior frontal gyrus (Aron et al. Reference Aron, Fletcher, Bullmore, Sahakian and Robbins2003). As the instructions pertain to inhibiting the motor response and not directly to suppression of the underlying thought, performance should be sensitive to unintentional suppression of thoughts accompanying an intentional action. This is an important distinction, as impairments in suppressing particular thoughts and memories may underlie the recurrent nature of obsessions.
The study further sought to address whether impaired inhibition would be found when inhibiting meaningful stimuli, as the standard stop signal task uses simple, non-emotive stimuli. An endophenotype of response inhibition would suggest impairment regardless of stimulus meaning. However, stimulus meaning appears to play a role in OCD performance in other forms of cognitive inhibition. For instance, in directed forgetting studies, OCD patients have difficulty suppressing and therefore forgetting certain words when instructed to do so, though results have been inconsistent as to whether this is exclusive to personally relevant negative material, all negative material, or personally relevant material regardless of valence (Wilhelm et al. Reference Wilhelm, McNally, Baer and Florin1996; Tolin et al. Reference Tolin, Hamlin and Foa2002; Bohne et al. Reference Bohne, Keuthen, Tuschen-Caffier and Wilhelm2005). Data from other paradigms using affective stimuli and believed to employ inhibitory processing such as negative priming, affective Stroop and thought suppression tasks have yielded inconsistent results (Hartston & Swerdlow, Reference Hartston and Swerdlow1999; Moritz et al. Reference Moritz, Jacobsen, Kloss, Fricke, Rufer and Hand2004). Further, the extent to which performance in these tasks requires inhibition is controversial (e.g. Tipper, Reference Tipper2001). Accordingly, the current study examined whether stimulus meaning influences response-related processing, inhibition-related processing, or both in patients with OCD.
Studies demonstrating response inhibition deficits in OCD using the stop-signal task have been careful to exclude co-morbidities. However, such co-morbidities are common (Pigott et al. Reference Pigott, L'Heureux, Dubbert, Bernstein and Murphy1994), with major depressive disorder (MDD) being the main one (Sasson et al. Reference Sasson, Zohar, Chopra, Lustig, Iancu and Hendler1997). In fact, little is known about the neuropsychological profile of OCD with concurrent depression. Some evidence suggests that OCD patients with co-morbid depression may have larger inhibitory deficits than those without depression, as the former exhibit additional brain abnormalities and differential metabolic responses to selective serotonin reuptake inhibitors (SSRIs) (Saxena et al. Reference Saxena, Brody, Ho, Alborzian, Maidment, Zohrabi, Ho, Huang, Wu and Baxter2002; Cardoner et al. Reference Cardoner, Soriano-Mas, Pujol, Alonso, Harrison, Deus, Hernandez-Ribas, Menchon and Vallejo2007). Nevertheless, response inhibition in patients with MDD and no OCD appears preserved (Murphy et al. Reference Murphy, Sahakian, Rubinsztein, Michael, Rogers, Robbins and Paykel1999; Lau et al. Reference Lau, Christensen, Hawley, Gemar and Segal2007), despite impairments in executive function, memory and affective processing (Chamberlain & Sahakian, Reference Chamberlain and Sahakian2004). Moreover, MDD in the context of OCD appears to differ from non-co-morbid depression in clinical features and treatment response (Fineberg et al. Reference Fineberg, Fourie, Gale and Sivakumaran2005). Hence we compared response inhibition in OCD patients with versus without co-morbid depression. Increased impairments to negative material would be expected, particularly in the former group, as individuals with MDD demonstrate increased sensitivity, slowed responding and enhanced memory for negative material (Chamberlain & Sahakian, Reference Chamberlain and Sahakian2004; Leppanen, Reference Leppanen2006).
In sum, we addressed the following questions using the TSST paradigm. First, when a motor response to a word is inhibited, is the accompanying thought suppressed, and if so, is this moderated by OCD? Given the important role of thought suppression in OCD (Rachman, Reference Rachman1998), it was of interest whether any evidence for abnormal unintentional thought suppression would be found. Second, is motor response inhibition in OCD influenced by the meaning of the stimulus triggering the go response? Third, are go responses influenced by stimulus meaning? OCD patients may demonstrate differential processing of OCD-relevant stimuli. Finally, do OCD patients with co-morbid depression demonstrate impaired response inhibition or thought suppression? The inhibitory dysfunction endophenotype would predict impaired response inhibition in OCD regardless of co-morbid depression.
Method
Participants
The study was approved by the local research ethics committee and all participants provided written, informed consent before testing. OCD patients (n=40), 20 with depression and 20 without depression, were recruited from a specialist OCD out-patient centre after being diagnosed and screened by a certified consultant psychiatrist (N.A.F.) using DSM-IV criteria (APA, 1994) and an extended clinical interview supplemented by the Mini-International Neuropsychiatric Inventory (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998). Though co-morbid anxiety and depression symptoms were not excluded provided OCD was the principal diagnosis, we excluded patients with other DSM-IV Axis-I co-morbidities, history of head injury or other neurological, neurodevelopmental or medically relevant disorders. OCD and depression severity were assessed with the Yale–Brown Obsessive Compulsive Scale (YBOCS; Goodman et al. Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989) and the Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979), respectively. There was a maximal MADRS cut-off of 10 for non-depressed patients and a minimal cut-off of 20 for depressed patients. Healthy controls (n=20), recruited via advertisements, scored 10 or below on the MADRS, and were screened for the exclusion criteria of psychiatric illness, history of head injury or neurological disorder and psychotropic medication. In the non-depressed OCD group, 19 patients were receiving SSRIs, of which ten were also receiving a low dose of an atypical neuroleptic, and one was medication free. In the depressed OCD group, 19 patients were receiving SSRIs of which one was also receiving a low dose of an atypical neuroleptic and one patient was medication free. Group characteristics and scores on the National Adult Reading Test (Nelson, Reference Nelson1982) assessing verbal intelligence, the Padua Inventory (Burns et al. Reference Burns, Keortge, Formea and Sternberger1996) characterizing self-reported compulsivity and obsessionality and the State-Trait Anxiety Inventory (STAI; Spielberger et al. Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983) assessing anxiety are reported in Table 1.
Table 1. Demographic and clinical measures from OCD depressed, OCD non-depressed and healthy control groups
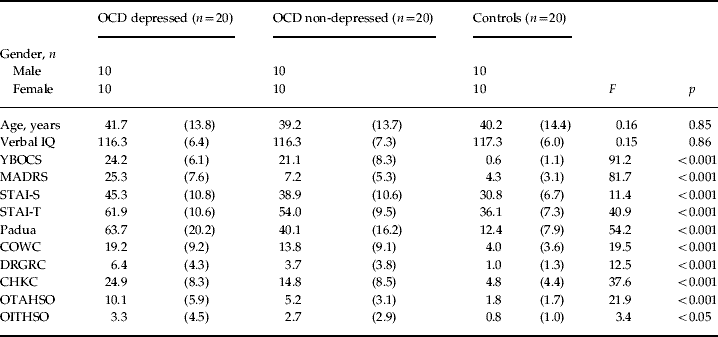
OCD, Obsessive–compulsive disorder; IQ, intelligence quotient; YBOCS, Yale–Brown Obsessive Compulsive Scale; MADRS, Montgomery–Asberg Depression Rating Scale; STAI, State-Trait Anxiety Inventory; -S, state; -T, trait; COWC, contamination obsessions and washing compulsions; DRGRC, dressing/grooming compulsions; CHKC, checking compulsions; OTAHSO, obsessional thoughts of harm to self/others; OITHSO, obsessional impulses of harm to self/others.
Values are given as mean (standard deviation).
TSST
The task comprised two blocks, one employing neutral words and the other employing OCD-relevant words, with presentation order counterbalanced within groups. In each block, a stop phase included 60 trials followed by a repetition phase of 80 trials (Fig. 1). On each trial following 500 ms fixation a word in white was presented on a black screen and participants pressed one of two response buttons determining whether the word was a noun or not (i.e. an adjective or verb) as fast as they could with their dominant hand. Mapping of responses to buttons was counterbalanced within each presentation order per group.

Fig. 1. Illustration of a typical series of trials in go no-repeat, go repeat and stop repeat conditions in the stop phase (a) and repetition phase (b) of the thought stop-signal task. For each word the participant makes a speeded response via a key press deciding whether the word is a noun or not. Go and stop repeat stimuli appear both in the stop and repetition phases while no-repeat stimuli are replaced with novel stimuli in the repetition phase. Stop signal delays in stop trials were determined online as a function of individual go reaction times (RTs). Stop repeat trials in the repetition phase were analysed based on stop outcome in the stop phase, with words that were previously inhibited successfully compared with responses to words that had previously failed to be successfully inhibited.
In the stop phase, participants were told that on occasion a tone will signal that they stop the ongoing task and refrain from pressing the buttons. Instead they should press another key with their non-dominant hand as fast as possible [switching to a different response, rather than simply stopping, was hypothesized to increase inhibitory effort (Logan & Burkell, Reference Logan and Burkell1986)]. In the stop phase, a third of trials were stop trials where the word was followed by a tone (440 Hz, 400 ms in duration) and was simultaneously replaced with a mask of random letters. The duration between word onset and tone onset, termed stop signal delay, was adjusted online individually to short or long (20% or 80% of median cumulative go trials duration). On the remaining go trials, a mask replaced the words with the corresponding short and long durations, but no tone was presented. Previous evidence suggests that repetition priming is sensitive to stimulus duration but not to shifting of responses during initial stimulus presentation (Logan, Reference Logan1985). The next trial began 3000 ms after stimulus onset, regardless of participant response.
In the repetition phase, no stop signals were presented and the words remained visible until response. All stop and go-repeat trials (randomly selected half of the go trials from the previous block equated for mask duration) were presented again with 40 new stimuli. The task was preceded by 18 practice go trials followed by a further 18 of which four were stop trials, with the order within each 18 randomized. Instructions were displayed at the onset of each phase and the experimenter ensured that they were understood. Self-terminated breaks were available between blocks and midway through each block.
OCD-relevant stimuli were drawn from OCD questionnaires and previous studies. Five clinicians and OCD researchers rated 258 words, of which the 100 judged most relevant were chosen. These included 29 contamination-related words (e.g. ‘toilet’), 15 checking-related words (e.g. ‘recheck’), 14 miscellaneous words (e.g. ‘blasphemy’), with the remaining words regarded as general to all OCD subtypes (‘worry’). The 100 neutral words were equated for word length, word frequency, and belonging to restricted semantic categories. Words were selected by two raters as likely to be perceived as neutral from the following categories: gardening (e.g. ‘shrub’), beach (e.g. ‘coastal’), fashion (e.g. ‘designer’) and office domains (e.g. ‘briefcase’), to match contamination, checking, miscellaneous and general domains, respectively. The task was programmed in visual basic.net (Microsoft Corp., USA) and stimulus order, condition order and allocation of words to condition were randomized for each participant. The task was administered on an Avantech Paceblade and go responses were performed on a custom button-box.
Task outcome measures included reaction time (RT) in ms and response chosen (noun or not a noun). Accuracy was not computed, as the majority of stimuli could be both nouns and adjectives/verbs. The data from the stop and repetition phases were analysed separately. Go trials with RTs faster than 400 ms and slower than 3000 ms consisted of 2.5% of all go trials and were omitted from the analyses (at the individual level a mean of 2.5% of trials was omitted, s.d.=3.2). Logarithmic mean go RTs and arcsine-transformed proportional data were analysed using analyses of variance (ANOVA) with simple main effects and Tukey's honest significant difference (HSD) for pairwise comparisons with an α level of 0.05 where appropriate. Data are presented as untransformed means. Pearson correlation coefficients were used for correlation analyses.
Results
Clinical and psychological rating scales
The three groups did not differ significantly with respect to age, gender or verbal intelligence quotient (see Table 1). Tukey's HSD comparisons revealed that the two OCD groups did not differ significantly in YBOCS overall or subscale scores (p>0.2) and the non-depressed OCD group did not differ significantly from controls on the MADRS score (p>0.24). For the STAI and Padua measures all comparisons between groups were significant, with the exception of the STAI-state scores between the two OCD groups. The Padua subscales indicated that all depressed OCD patients had prominent checking compulsions and all but one had contamination obsessions and compulsions. All but one of the non-depressed OCD patients had prominent checking compulsions and all but two had contamination obsessions and compulsions. Omitting these patients did not alter the results reported below.
Analysis of stop phase
Go RT responses to the words were analysed with group (OCD depressed, OCD non-depressed and controls) and stimulus type (OCD-relevant versus neutral) as factors. Latencies for button presses to OCD-relevant stimuli (1015 ms) were longer than for neutral stimuli (957 ms). The ANOVA revealed a significant effect for stimulus type [F(1, 57)=6.3, p<0.05], with all other effects failing to reach significance (p>0.32 for all).
Inhibition performance was examined by analysing the proportion of successfully inhibited trials with group as a between-subjects factor and stimuli type and delay (short versus long) as repeated measures. The proportion of successfully inhibited trials for the OCD depressed (0.48) and non-depressed groups (0.50) did not significantly differ (p=0.97) but were both significantly worse than for the control group (0.75) (Cohen's d=0.76) (see Fig. 2). As anticipated, the inhibition proportion was higher for short delays (0.84) than for long delays (0.29). Correspondingly, an ANOVA revealed a significant effect for group [F(2, 57)=6.4, p<0.01], delay [F(1, 57)=244, p<0.01], and for the interaction between stimulus type and delay [F(2, 57)=7.5, p<0.05]. The remaining effects were not significant (p>0.34 for all). The interaction between stimulus type and delay stemmed from a crossover with OCD stimuli leading to lower inhibition than neutral stimuli in the short delay (0.83 v. 0.85, respectively), but higher inhibition in the long delay (0.32 v. 0.26, respectively). No simple main effect reached significance.

Fig. 2. Percentage successful inhibition to the stop signal following obsessive-compulsive disorder (OCD)-relevant (![]() ) and neutral (□) stimuli in OCD patients with co-morbid depression (OCD+dep), OCD patients without co-morbid depression (OCD−dep) and a control group. Values are means, with standard errors represented by vertical bars. The figure demonstrates impaired inhibition in the OCD groups regardless of stimulus type.
) and neutral (□) stimuli in OCD patients with co-morbid depression (OCD+dep), OCD patients without co-morbid depression (OCD−dep) and a control group. Values are means, with standard errors represented by vertical bars. The figure demonstrates impaired inhibition in the OCD groups regardless of stimulus type.
Switch performance following a stop signal was examined by analysing RTs to press the space-bar following successful stops (see Fig. 3). A 3×2 ANOVA with group and stimulus type revealed a main effect for stimulus type [F(1, 57)=9.3, p<0.01] and interaction between group and stimulus type [F(2, 57)=4.2, p<0.05] with no effect for group (p>0.5). Simple main effects indicated that there was a significant stimulus type effect [F(1, 57)=16.0, p<0.01] that did not differ between the two OCD groups (p>0.4), but no stimulus type effect for the control group (p>0.45).
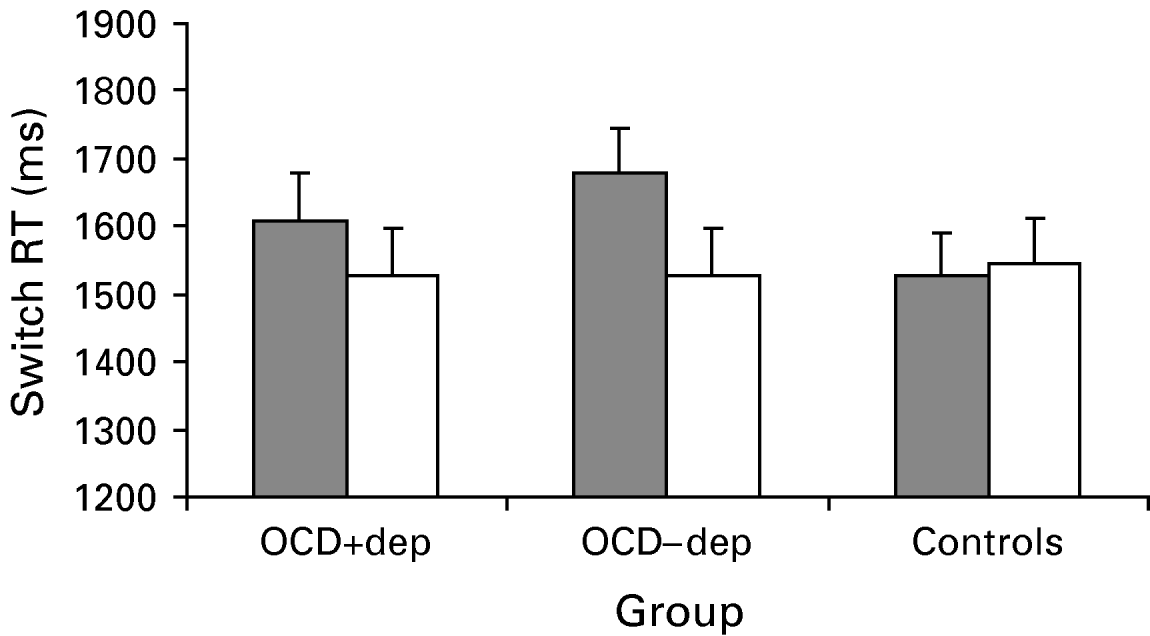
Fig. 3. Reaction time (RT) performance to pressing the space bar following a stop signal following obsessive-compulsive disorder (OCD)-relevant (![]() ) and neutral (□) stimuli in OCD patients with co-morbid depression (OCD+dep), OCD patients without co-morbid depression (OCD−dep) and a control group. Values are means, with standard errors represented by vertical bars. The figure demonstrates slowed switching in the OCD groups which is specific to OCD-relevant stimuli.
) and neutral (□) stimuli in OCD patients with co-morbid depression (OCD+dep), OCD patients without co-morbid depression (OCD−dep) and a control group. Values are means, with standard errors represented by vertical bars. The figure demonstrates slowed switching in the OCD groups which is specific to OCD-relevant stimuli.
Thus, results revealed impaired motor response inhibition in OCD and an effect for stimulus type on switching that was exclusive to OCD patients. At the same time all participants showed similar go RT slowing to OCD-relevant stimuli.
Analysis of repetition phase
Mean go RTs in the repetition block were subjected to an ANOVA with group as a between-subjects factor and stimulus type and repetition condition (repeat go, repeat stop and novel stimuli) as within-subjects factors. As in the stop block, RTs to OCD-relevant stimuli (1022 ms) were significantly slower than to neutral stimuli (968 ms) [F(1, 57)=17.6, p<0.01]. In addition, there was a repetition priming effect [F(2, 57)=14.8, p<0.01], with RTs to repeat go stimuli (975 ms) and RTs to repeat stop stimuli (982 ms) being significantly faster than to novel stimuli (1026 ms, p<0.05). RTs to repeat go and repeat stop did not significantly differ (p>0.5). To test thought suppression, RTs to repeat stop trials were entered into an additional ANOVA with group as a between-subjects factor and stimulus type and stop outcome as within-subjects factors. Stop outcome refers to whether the stop response to the stimulus in the stop block failed or was successful. RTs to stimuli of successful stops were significantly longer (1015 ms) than those of failed stops (924 ms) [F(1, 55)=25.3, p<0.01]. All other effects with the exception of stimulus type were non-significant (all p>0.35).
Although absolute accuracy could not be analysed, consistency across presentations was examined. Proportion consistent refers to the proportion of stimuli to which participants responded with the same judgement in both the stop and repetition phases. An ANOVA on proportion consistent including group and stimulus type revealed a main effect of group [F(2, 57)=4.2, p<0.05], with all other effects being non-significant (p>0.15 for all effects). Proportion consistent for the OCD depressed (0.80) and non-depressed groups (0.81) did not significantly differ (p>0.5) but was worse for both OCD groups than for controls (0.88, Cohen's d=0.81). The comparisons between OCD depressed and controls and OCD non-depressed and controls were both significant (p<0.02).
Thus, these results indicate intact thought suppression as measured by repetition priming in OCD patients, though less consistent responses.
Correlation analyses
Correlations between MADRS, YBOCS, STAI-trait and Padua scores were all significant within the combined sample of OCD patients (Pearson's r values ranging from 0.39 to 0.61, p<0.05). Correlations between TSST-related measures and symptom severity measures demonstrated a significant correlation between the slowing to OCD compared with neutral stimuli in the repetition phase, and MADRS scores [r=0.38, t(38)=2.5, p<0.05]. When examining the Padua subscales there were significant negative correlations between stopping performance to neutral words and the contamination obsessions and washing compulsions scale [r=−0.33, t(38)=2.0, p<0.05], and stopping performance to concern words and obsessional impulses to harm self or other [r=−0.33, t(38)=2.2, p<0.05]. There were no significant correlations between stop and switch performances.
Discussion
This study tested whether OCD patients with or without depression differed from controls in a novel adaptation of the TSST paradigm. The task required participants to decide whether words were nouns or not and on occasion a tone signalled that they were to inhibit their response and instead switch to a different one. Repetition priming on a subsequent presentation gauged thought suppression. OCD patients demonstrated impaired motor inhibition compared with controls for all stimulus types, and impaired switching from OCD-relevant but not neutral words. Stimulus meaning did not differentially influence patient stop or go performances per se, nor did it affect the subsequent processing of the words as measured by repetition priming. These results indicate that emotional relevance of the stimulus interacts differentially with the various executive components in the TSST paradigm, i.e. inhibition and switching, in OCD patients. The findings also reinforce the potential usefulness of response inhibition dysfunction across different contexts as an endophenotype for OCD (Chamberlain et al. Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005), especially as the deficits in OCD were found regardless of depression status. Inhibition performance within the patients also correlated with particular subscales of the Padua Inventory, suggesting that patients who experience contamination obsessions or impulses to harm themselves or others may be especially impaired. The results extend previous stop-signal findings in OCD, as impaired stopping was found with considerably more demanding go judgements than previous stop signal tasks which used simple spatial discriminations, and which did not require response switching.
All groups were slower to respond (go RT) to the OCD-relevant words, with no differential slowing in the OCD groups. Prior work has shown that medicated OCD patients do not show abnormal RT bias to generally affective stimuli (Chamberlain et al. Reference Chamberlain, Fineberg, Blackwell, Clark, Robbins and Sahakian2007a), though there may be abnormal neural processing (Menzies et al. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008). Whereas stimulus meaning had no differential effect on response generation and inhibition, it did have a clear effect on the speed of an alternate, switching response. Impaired switching in the TSST may be attributed to dysfunction in several potential mechanisms, including disengaging from the go stimulus, shifting, or responding to the tone (Posner & Petersen, Reference Posner and Petersen1990). The lack of significant differences between the groups' performance in the repetition phase suggests that impaired switching in OCD was primarily due to shifting or re-engaging with a different stimulus. This is consistent with evidence that OCD patients have difficulties in attentional set-shifting and exhibit cognitive inflexibility (Chamberlain et al. Reference Chamberlain, Fineberg, Blackwell, Robbins and Sahakian2006a). Moreover, the current results indicate that stimulus meaning may worsen such impairments. Stimulus salience or relevance may play a role in accounting for inconsistencies in the OCD literature as in the case of the Wisconsin Card Sorting Test (Kuelz et al. Reference Kuelz, Hohagen and Voderholzer2004) and directed forgetting (see below). Future studies may further explore the role of stimulus relevance in cognitive shifting, switching and flexibility in OCD. OCD patients were also less consistent when judging the same stimulus again, i.e. they were less likely than controls to choose the same response on repetition trials. This may reflect worse memory, though we believe it is more likely to be attributable to general indecision, or impaired stimulus-response learning as often found in OCD patients (Muller & Roberts, Reference Muller and Roberts2005).
As predicted, there was a difference in the repetition phase between previously successfully stopped and previously failed stop words, confirming the existence of thought suppression. Thus, repetition priming was indeed sensitive to the fate of the mental processing accompanying the word judgement, suggesting that when a judgement was successfully inhibited, the accompanying thought was abandoned. These effects occurred equally in all groups, supporting intact unintentional thought suppression in OCD in the repetition phase, and limiting the ‘inhibition endophenotype’ for OCD to volitional motor responses. Thus, the OCD groups were not impaired in disengaging from the word stimulus although they were impaired at switching from OCD-relevant words in the initial phase. This suggests that they did not ruminate unnecessarily or carry out the judgement implicitly whilst complying overtly with task instructions. That individuals with OCD appear to terminate their ongoing processing effectively when given a salient external signal to switch may be useful for various forms of behavioural therapy. For example, rather than identifying and monitoring their current obsessions internally, severe OCD patients may initially be externally cued on occasion to facilitate their refocusing away from ongoing obsessions.
The lack of effect of co-morbid depression on TSST performance measures is consistent with the response inhibition endophenotype hypothesis. Namely, inhibitory deficits were not exacerbated by the individual's current affective state. Although the OCD groups were matched for symptom severity in clinician-rated YBOCS scores, depressed OCD patients scored higher in all self-report measures including anxiety, obsessions and compulsions. This dissociation between self-report and TSST performance further illustrates the importance of stable, objective and replicable markers of dysfunction in OCD. Moreover, the results strengthen the notion that depression within the context of OCD differs from depression without OCD, as no abnormal processing of affective stimuli was noted. While the present study cannot generalize to individuals with MDD and no OCD, it is generally not incompatible with evidence that such individuals have preserved response inhibition (Lau et al. Reference Lau, Christensen, Hawley, Gemar and Segal2007). Preserved response inhibition in MDD could be important as it stands in contrast to the broad impairments in executive function typically found (Chamberlain & Sahakian, Reference Chamberlain and Sahakian2004).
Impairments in inhibition have been reported in several additional psychiatric disorders to date, including attention deficit hyperactivity disorder (ADHD) and schizophrenia (Badcock et al. Reference Badcock, Michie, Johnson and Combrinck2002; Lijffijt et al. Reference Lijffijt, Kenemans, Verbaten and van Engeland2005) and thus are not specific to OCD. Nevertheless, response inhibition deficits in OCD appear integral to aspects of the symptoms and psychology of OCD (Chamberlain et al. Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005). Moreover, the exact nature of the inhibitory deficits and the developmental trajectory associated with each disorder appear different. In the case of ADHD, impaired response inhibition seems more pronounced in adult ADHD (Lijffijt et al. Reference Lijffijt, Kenemans, Verbaten and van Engeland2005). The exact pattern of impaired inhibition across the life span in OCD is as yet undetermined, but the endophenotype hypothesis makes clear predictions about its presence before symptom onset. By using more refined tests of inhibition, a better characterization of inhibitory impairments in each disorder may be afforded. Stimulus meaning may be used to further probe inhibitory performance across various disorders.
One limitation of the present study is that almost all patients were stabilized on SSRI medication at testing, which could have led to diminished emotional responses to OCD-relevant words (Harmer et al. Reference Harmer, Shelley, Cowen and Goodwin2004). Accordingly, medication status and responsiveness may underlie inconsistent results as with affective Stroop in OCD (Kuelz et al. Reference Kuelz, Hohagen and Voderholzer2004). Future studies could explore medication influences on emotional processing in OCD by studying unaffected first-degree relatives. SSRI medication in the OCD groups did not, however, mask the response inhibition deficit, in keeping with findings that serotonergic manipulations do not influence stop-signal performance (Clark et al. Reference Clark, Roiser, Cools, Rubinsztein, Sahakian and Robbins2005; Chamberlain et al. Reference Chamberlain, Muller, Blackwell, Clark, Robbins and Sahakian2006b). Another limitation is the inclusion of OCD-relevant stimuli with different valences and encompassing various obsessionality and compulsivity domains. Prior work has yielded conflicting results as to whether OCD patients show impairments to primarily OCD-relevant stimuli regardless of valence, or primarily to negative valence regardless of OCD-relevance (Tolin et al. Reference Tolin, Hamlin and Foa2002; Bohne et al. Reference Bohne, Keuthen, Tuschen-Caffier and Wilhelm2005). Whilst the OCD-relevant stimuli were mostly negative, owing to the large number of stimuli required, we included words with potentially positive valence such as ‘health’ and ‘certain’. We believe that all words in the OCD-relevant block would be interpreted within the general context of negative OCD-relevant stimuli. Likewise, for each participant the majority of words would be relevant to their particular concerns, as over half the stimuli were selected as relevant to all OCD domains. Accordingly, all participants identified the OCD-relevant block as more negative.
In conclusion, the results support inhibition deficits that are not influenced by stimulus meaning in OCD patients with or without co-morbid depression. For all groups, word processing stopped with action termination, regardless of word meaning. The study has indicated that the endophenotype of response inhibition in OCD (Menzies et al. Reference Menzies, Achard, Chamberlain, Fineberg, Chen, del Campo, Sahakian, Robbins and Bullmore2007) can be extended to patients with co-morbid depression and to meaningful stimuli, though not to unintentional thought suppression.
Acknowledgements
The authors thank the volunteers who took part. This study was funded by a Wellcome Trust Programme Grant (076274/Z/04/Z) to B.J.S. and T.W.R. The Behavioural and Clinical Neuroscience Institute is supported by a joint award from the Medical Research Council and Wellcome Trust.
Declaration of Interest
B.J.S. and T.W.R. consult for Cambridge Cognition and a number of pharmaceutical companies. They also hold shares in CeNeS.





