INTRODUCTION
Surgical site infections (SSIs) are a common hospital-acquired infection. In Germany, SSI has been shown to be the third most frequent nosocomial infection with a prevalence of 16% [Reference Ruden1] and the second most frequent in the USA with a prevalence of 24% [Reference Haley2]. Patients who develop SSI usually stay longer in hospital, have an increased mortality rate, and contribute to increased costs [Reference Kirkland3, Reference Scott4]. The German surveillance system for nosocomial infections (Krankenhaus-Infektions-Surveillance-System; KISS) showed that the prolongation of hospital stay due to SSI was 13·4 days, twice as long as due to nosocomial pneumonia (6·2 days) [Reference Beyersmann5]. In a large study involving more than 60000 patients in England, Coello and colleagues reported that prolongation of hospital stay due to SSI was 12·2 days and was associated with 10·2 deaths/100 operations, with attributable costs of between £959 and £6103 per patient [Reference Coello6]. A more recent study from Switzerland found a mean additional hospital cost of CHF 19638 [Reference Weber7].
The type of surgery influences the frequency of SSI. Coello et al. [Reference Coello6] reported an incidence of 7·7% in vascular surgery while Richet et al. [Reference Richet8] found a higher incidence of SSI after vascular surgery of the lower extremities (7·8%), compared to all vascular surgical procedures (3·4%). Indeed, the incidence of SSI doubled in patients with implantation of synthetic prosthesis compared to those that received autologous grafts [Reference Szilagyi9]. SSI contributes significantly to morbidity and mortality in vascular surgery, particularly after implantation of biografts or synthetic prosthesis [Reference Calligaro10, Reference Calligaro11] and mortality rates of 14–26% following graft infection have been reported after aortic bypass surgery [Reference Batt12, Reference Bisdas13].
Few studies investigating risk factors for SSI following vascular surgery are available. Given the high impact on cost, morbidity and mortality of these infections in vascular surgery, we undertook a retrospective cohort study to determine the SSI rate and to perform a risk factor analysis for SSI in a cohort of patients with vascular surgical procedures.
MethodS
We conducted a retrospective cohort study at the Hannover Medical School, a 1400-bed university hospital. All patients who received arterial vascular surgery below the aortic arch (thoraco-abdominal, abdominal, retroperitoneal, iliaco-femoral, femoral, femoro-distal) between 1 January 2002 and 31 December 2005 were considered for the study. Patients with pre-existing infections at the operating site before operation were excluded. For data collection we reviewed clinical inpatient and outpatient charts, medical notes, and operating room records. SSI was determined according to the Centers for Disease Control and Prevention (CDC) definitions, and subdivided into superficial, deep, and organ/space [Reference Horan, Andrus and Dudeck14].
Preoperative, perioperative and postoperative characteristics from patients were collected. Preoperative characteristics included demographic data (age, sex), body mass index (BMI), and comorbidities. Comorbidities were defined as diseases that did not lead to surgery directly and thus there were two groups of patients with peripheral atherosclerotic disease (PAD): those that were operated due to PAD, thus PAD was the indication for surgery, and those that were operated for another reason (e.g. embolus formation, vascular rupture) but had PAD as a comorbidity. Physical status according to American Society of Anesthesiologists (ASA) criteria was also considered as a preoperative characteristic [15]. Further preoperative characteristics were anticoagulant prophylaxis prior to admission, smoking status, previous vascular surgery at the same operating site, immunosuppression (due to therapy), indication for surgery, and blood transfusion. Perioperative characteristics included perioperative antibiotic prophylaxis (PAP), surgical access, surgical techniques, duration of operation, type of prosthesis (patches, stent grafts, bypasses), complexity of surgery (e.g. single vs. multiple procedures during the same session), use of a heart-lung machine, and blood transfusion. Postoperative characteristics included duration of postoperative drainage, development of haematoma, length of hospital stay, and blood transfusion. In all 119 parameters were processed in a bivariable analysis. For all patients with SSI, the microbiological records were searched for identification of bacterial or fungal pathogens and resistance profile.
Statistical analysis
The overall SSI rate with 95% confidence intervals was calculated. The Wilcoxon rank sum test was used to compare hospital stay of patients with and without SSI. Patients' characteristics were transformed into binary variables to describe risk factors for the development of SSI. Unadjusted relative risks and 95% CIs for SSI were determined, and a Fisher's exact test (two-sided) was used to test the association between risk factor and the development of SSI. A multiple logistic regression analysis with stepwise variable selection (forward and backward) was performed with SSI as the outcome. Patients with a calculated BMI (n=747) were included in the multivariable analysis. Adjusted odds ratios (aORs) and 95% CIs for risk factors in the logistic regression model were calculated. The significance level for entering a variable into the model and for removing a variable from the model was 0·05. Statistical analysis was performed using SAS statistical software version 9.2 (SAS Institute Inc., USA). P<0·05 was considered significant.
Results
During the observation time period, inclusion criteria for this study was met by 756 patients and SSIs occurred in 44 (5·8%, 95% CI 4·3–7·7). Patients who developed SSI remained significantly longer in hospital after vascular surgery than those without SSI (mean 21 days vs. 11 days, P<0·001). Of the 44 SSI cases, 29 were superficial, five were deep, and 10 were organ/space SSI (seven graft infections). On average SSI developed 17 days (range 1–126 days) after surgery. Superficial infections occurred earliest (mean 14 days), followed by deep (mean 20 days), and organ/space (mean 26 days) SSIs.
The principal surgical access was an inguinal incision, followed by median laparatomy, and thoraco-retroperitoneal access. More than half of SSIs occurred in patients with inguinal access, 25% after abdominal access and 14% after thoraco-retroperitoneal access (Table 1). Of the 756 patients in the study, 684 (91%) received PAP. Of these, 13 had received the first intravenous dose of the antibiotic more than 120 min prior to incision, 178 patients 120 to 30 min prior to incision, 413 patients 30 to 0 min prior to incision, and 80 patients after incision during the operation.
Table 1. Surgical site infection (SSI) following different surgical access
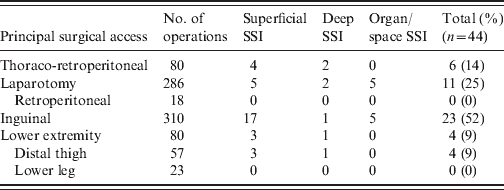
The results of microbiological cultures were available for 40/44 patients with SSI. Two or more pathogens were isolated in 26 (59%) cases; the most common were coagulase-negative staphylococci (22%), followed by Enterococcus spp. (20%), Staphylococcus aureus (18%), Pseudomonas aeruginosa (10%), Escherichia coli (7%), and Candida spp. (5%).
Table 2 shows all parameters that were significantly associated with SSI by bivariable analysis. Preoperative characteristics included >6 days length of hospital stay prior to operation, a BMI >29, the comorbidities PAD, renal failure, immunosuppression, and an ASA score >2. Of indications for surgery, PAD (Fontaine stages III–IV) was most associated with SSI. Among perioperative characteristics femoral grafting, duration of operation >240 min, utilization of autologous veins and/or artery, and the insertion of more than two drainage tubes during operation were significantly associated with SSI. In the postoperative period, the presence of drainage tubes for >5 days, and development of haematoma were significantly associated with increased risk of developing SSIs.
Table 2. Bivariable analysis of investigated factors increasing or decreasing relative risk for surgical site infection following vascular surgery in 756 patients

n.d., Not defined; RR, relative risk; CI, confidence interval; PAD, peripheral atherosclerotic disease; ASA, American Society of Anesthesiologists.
* Fisher's exact test.
† Body mass index could be calculated for 747 patients.
‡ Multiple indications may apply for single patients.
§ Multiple procedures during the same session.
¶ Embolectomy, endarterectomy, percutaneous transluminal angioplasty.
Parameters significantly associated with SSI in the bivariable analysis were subjected to multivariable analysis. Significant risk factors after adjustment by multiple logistic regression analysis were femoral grafting, PAD (Fontaine stages III–IV), postoperative drainage for >5 days, immunosuppression, duration of operation >214 min, and BMI >29 (Table 3). The application of PAP was independently associated with a decreased risk of SSI (Table 3).
Table 3. Multivariable analysis of factors associated with increased or decreased risk for surgical site infection following vascular surgery in 747 patients
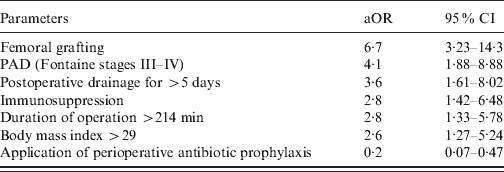
aOR, Adjusted odds ratio; CI, confidence interval; PAD, peripheral atherosclerotic disease.
c-Index for multivariable analysis was 0·829.
Using stent grafts by minimal invasive surgery could not be subjected to multivariable analysis for mathematical reasons. No patient receiving a stent graft by minimal invasive surgery developed a surgical site infection.
The application of stent graft by minimal invasive surgery was significantly associated with a decreased risk for SSI (P=0·004) in the bivariable analysis. However, as none of the patients with stent grafts by minimal invasive surgery developed a SSI, application of stent grafts could not be subjected to the multivariable analysis and a 95% CI could not be provided (Table 2).
Discussion
As expected, Gram-positive bacteria dominated the spectrum of pathogens isolated from SSIs in this study (67·4%). Whereas S. aureus was the most frequently isolated Gram-positive organism in other studies [Reference Lee16], here, this species was ranked third among all organisms.
Several studies have reported incidences of SSI following vascular surgery of between 2·4% and 8·5% [Reference Leong, Wilson and Charlett17–Reference Geubbels20] and thus the incidence of 5·8% in our patient cohort is within the expected range. Notable independent risk factors for SSI were PAD (Fontaine stages III–IV), and obesity (BMI >29). The latter has been reported as an independent risk factor for the development of SSI after colorectal, gynaecological, cardiac [Reference Harrington21–Reference Myles, Gooch and Santolaya23] and vascular surgery [Reference Lee16, Reference Turtiainen24]. Similarly, PAD has been linked with increased risk of SSI after vascular surgery [Reference Earnshaw25].
The rate of SSI following inguinal access varies between 8% and 38% [Reference Lee16, Reference Chang19, Reference Hassen and Fitridge26]. In the present study femoral grafting was identified as an independent risk factor for SSI (aOR 6.7). Femoral grafting involves complex surgery, exposing the operation site to nearby contaminated areas such as the perineum which possibly explains the increased risk of infection. Richet and colleagues also found femoro-femoral bypasses to be associated with an increased risk of SSI but failed to confirm independence of the risk factor in subsequent multivariable analysis [Reference Richet8].
Until recently, previous studies have failed to find immunosuppression as an independent risk factor for the development of SSI [Reference Richet8, Reference Lee16, Reference Greenblatt, Rajamanickam and Mell27]. However, as was also demonstrated in our series, Konishi et al. showed that preoperative administration of steroids was independently associated with SSI following rectal procedures [Reference Konishi28]. Moreover, immunosuppression due to steroids was also associated with SSI in patients undergoing general and vascular surgery [Reference Neumayer29].
A longer duration of surgery is a well established risk factor for postoperative infection and estimates suggest that the infection rate of aseptic surgical procedures approximately doubles every hour of the procedure [Reference Cruse and Foord30]. Chang et al. reported an increased risk for SSI in patients and vascular surgery of the lower extremities after 318 min duration [Reference Chang19] and speculated, that prolonged operation times reflect other factors such as the technically demanding procedures of distal surgery. Therefore, in our study we included a broad range of surgical techniques involving several operation sites, and nonetheless found that duration of operation for more than 214 min was independently associated with risk of SSI (aOR 2.8).
The influence of the duration of postoperative surgical drainage on the development of SSI is not clear. Several studies have demonstrated that following both abdominal and orthopaedic surgery, postoperative drainage was associated with an increased rate of SSI [Reference Pessaux31, Reference Parker, Roberts and Hay32] but did not include duration of drainage. Here, postoperative drainage >5 days was clearly associated with SSI and this finding underlines the necessity of adequate homeostasis during surgery to avoid the need for drains in the first place and to minimize the duration of retention of the drain.
Besides risk factors, we found protective factors such as PAP and application of stent grafts by minimal invasive surgery. Several randomized studies have shown that PAP significantly reduces SSI rate in patients after vascular surgery [Reference Pitt33, Reference Worning34]. However, to date no study has shown it to be an independent protective factor in patients undergoing vascular surgery. PAP was also found here to be independently associated with protection against SSI (aOR 0.2), with an 80% reduction in the odds of developing a SSI for those receiving PAP. The majority (91%) of patients received PAP, demonstrating good compliance of medical practice in our clinic, but a more detailed analysis revealed that PAP was applied in only 178 (30%) patients within the recommended time-frame of 120 to 30 min before incision. Thus its benefit might be further increased by adhering to the recommended protocol, as it has been shown that application of PAP more than 2 h before incision or more than 3 h after incision significantly increases the rate of SSI compared to its administration 120 to 30 min before incision [Reference Classen35, Reference Weber36].
An intriguing finding of our study was the lack of SSIs in 92 patients treated with stent grafts by minimal invasive surgery, which could not be included in the multivariable analysis. Nonetheless, implantation of stent grafts by minimal invasive surgery turned out to be a protective factor in bivariable analysis. Several studies have examined the morbidity, including SSI, and mortality following implantation of stent grafts in vascular surgery patients [Reference Slappy37–Reference Torsello39] but none had performed a risk factor analysis. Based on our results, we believe that the utilization of stent grafts may be considered, for example, in patients with non-modifiable risk factors such as obesity. It has been shown by Chuter and colleagues that both morbidity and mortality are significantly reduced when obese patients were provided with stent grafts instead of receiving conventional reconstructive surgery [Reference Chuter40].
A limitation of our study is the lack of systematic post-discharge surveillance for patients who did not develop a SSI during their hospital stay. Patients were followed throughout their hospital stay, and occurrence of SSIs were recorded. Additional SSIs after discharge were detected if patients returned to our facility as outpatients (n=279). Of the 44 cases with SSI, 42 developed SSI during their initial hospital stay, and two cases were reported from the outpatient clinic. Thus the total number of SSI cases in our study population might be underreported.
In summary, we found that femoral grafting, PAD (Fontaine stages III–IV), immunosuppression, and obesity were independent risk factors for the development of SSI following vascular surgery. As the ability to influence these factors is limited, compliance with well established prevention measures for reduction of SSIs is of particular importance to reduce their incidence in these patients. Our findings also suggest that duration of operation and duration of postoperative surgical drainage, two other independent risk factors, should be kept as short as possible. PAP was associated with increased protection against SSI, and should be a standard procedure in clean vascular surgery when grafting is involved.
DECLARATION OF INTEREST
None.




