Introduction
Entomology is a branch of science that has been practiced in Canada for a long time. Three early indications of this were the foundation of the Entomological Society of Canada in 1863, the publication of the first volume of The Canadian Entomologist in 1869 (Timms Reference Timms2009), and foundation of the Entomological Society of British Columbia in 1901 (Glendenning Reference Glendenning1933). However, entomological work by amateur naturalists pre-dated the formal establishment of the Canadian entomological societies (Alfaro Reference Alfaro1985). Because of the rich connection of Canadians to forests, forest entomology is also an old science. Generations of forest entomologists have made an enormous contribution to this field as described in the papers in this special issue; however, much has changed in Canadian forest entomology over this long history, and continued change is inevitable.
In this paper we briefly review the major changes in forest entomology in Canada, and provide a perspective regarding the future of this branch of entomology.
Changing paradigms
Before the 1930s, forest entomology in Canada focussed mostly on natural history studies, in which Canadian entomofauna was described and catalogued, and in some cases basic natural history was elucidated (e.g., see Aukema Reference Aukema, McKee, Wytrykush and Carroll2016). Given the large territory and complex ecosystems of Canada, the descriptive phase continues even today, albeit with new tools, such as molecular techniques (e.g., Lumley and Sperling Reference Lumley and Sperling2011).
However, over the decades, and in response to several drivers of change, such as societal pressures for a clean environment and for a sustainable management of forests, plus new technological advances, the focus of research and development in forest entomology has experienced a number of abrupt changes or paradigm shifts. These phases are the main subject of the next sections. However, Holmes and MacQuarrie (Reference Holmes and MacQuarrie2016) reviewed the forest protection phase so it is only briefly described here.
The forest protection phase
With the development of a strong forest industry starting in the 1930s, forest entomology research evolved to provide the biological and technical knowledge required to protect Canadian forests from pests (Prebble Reference Prebble1975). Forest protection was perceived to be important to maintain the supply of fibre for domestic and international markets, thereby maintaining the competitive status of the Canadian forest industry and the livelihoods of those working in the forest sector. Insecticide development and application, especially aerial application of chemical and biological (microbial) pesticides, was an important protection tool that was the centre of much research and development during this period (Holmes and MacQuarrie Reference Holmes and MacQuarrie2016). Forest protection required knowledge and technology for development and testing of new control products to improve spray application technology, including the influences of meteorology on spray operations, with the later being important to minimise pesticide drift and harm to non-target organisms. Entomology work focussed on life-stage studies of target and non-target organisms in relation to pesticide application, in order to maximise control efficacy while reducing impacts on non-target organisms. The forest protection phase is also characterised by a strong focus on classical biological control with the peak research activity and application in the 1930s, 1940s, and 1950s (MacQuarrie et al. Reference MacQuarrie, Lyons, Seehausen and Smith2016).
The emergence of integrated forest pest management
The 1970s saw the full impact of pivotal publications, such as Rachel Carson’s Silent Spring (Carson Reference Carson1962). In her influential book, Carson described the problems associated with indiscriminate use of pesticides for crop protection, and in particular the negative impacts of the large aerial spray operations, which were undertaken, starting in the 1950s, over forests of the United States of America and Canada. In a dramatic description (see Carson’s Chapter 10: Indiscriminately from the skies), she raises the alarm regarding the harmful impacts of pesticides, such as dichlorodiphenyltrichloroethane (DDT), on non-target organisms, biodiversity in general, livestock, and human health. Her book strongly influenced the decisions to ban DDT application and the development of stronger regulations on broadcast chemical pesticide application. The subsequent decline in reliance on chemical application to control pests created a demand and opportunities for new approaches for pest management in forestry and agriculture.
In the 1970s, in response to societal pressures for new pest management approaches, agricultural entomologists responded with the development of a new concept, integrated pest management (IPM). One widely accepted definition of IPM is: “an ecosystem-based strategy that focuses on long-term prevention of pests or their damage through a combination of techniques such as biological control, habitat manipulation, modification of cultural practices, and use of resistant varieties. Pesticides are used only after monitoring indicates they are needed according to established guidelines, and treatments are made with the goal of removing only the target organism. Pest control materials are selected and applied in a manner that minimizes risks to human health, beneficial and non-target organisms, and the environment” (University of California, Davis 2015).
The new IPM paradigm had a large appeal among policy makers, scientists, environmental organisations, and the public at large. In both the United States of America and Canada, the move towards IPM commenced in the 1960s and became firmly established in the late 1970s and 1980s.
The inclusion of IPM in the curricula of North American universities and the education of a new generation of professionals versed in the principles of IPM was critical in its rapid adoption. In 1967, Simon Fraser University, in Burnaby, British Columbia, Canada, established the Pest Management Centre, which became the first structured professional programme leading to the then-novel degree of Master in Pest Management. This programme graduated many Canadian and international “pest managers”, with most finding employment in provincial and federal establishments and the private sector.
Integrated pest management developed into an approach that incorporated various pest control and management methods into a rational and integrated programme, which was seen not as a single pest control solution, but rather an iterative series of actions, including pest population and damage evaluations, decision making, and the application of multiple control strategies and tactics. In practicing IPM, agricultural growers and forest managers were encouraged to follow a four step approach, as described by the Environmental Protection Agency (2015) of the United States of America for agricultural crops:
-
1. Establishing action thresholds: before taking any pest control action, IPM first sets an action threshold, defined as a point at which pest populations or environmental conditions indicate that a pest control action must be taken. The determination of the level at which pests will become an economic threat is critical to guide future pest control decisions.
-
2. Identifying and monitoring of pests: precise identification of the pest is essential, as damaging species co-occur with many innocuous or even beneficial organisms. Integrated pest management programmes prioritise the continuous monitoring of pest levels, so that appropriate control decisions can be made by comparing the current or projected pest population level with action thresholds. This monitoring and identification removes the possibility that pesticides will be used when they are not really needed.
-
3. Prevention: as a first line of defence, IPM programmes seek to manage the crop to prevent pests from becoming a threat. In forestry, this means using silvicultural methods, such as manipulating species mix and stem density, planting pest-resistant varieties, and treatment of infested patches. These tactics can be effective and cost-efficient, and present lower risk to people and the environment compared with large scale chemical pesticide application.
-
4. Control: once these earlier steps indicate that an action threshold has been reached, then pest control measures are required, as further preventive methods are no longer effective or available. The control phase of IPM must first evaluate the full suite of control strategies and tactics available to ensure that the most promising approaches that balance effectiveness and risk are implemented first. Effective and less-risky control options are chosen first, including highly specific chemicals such as pheromones to disrupt pest mating or protect trees, mechanical removal of infested trees, and trapping. If further monitoring indicates that less risky controls are not working to reduce pest populations and damage below action thresholds, additional pest control methods are then employed, such as targeted spraying of pesticides. Broadcast spraying of non-specific chemical pesticides is considered a last resort in IPM.
Integrated pest management in Canadian forests
Forest entomologists in Canada quickly adopted and promoted the concepts of IPM. In 1977, the Canadian federal government created the Forest Pest Management Institute, in Sault Ste. Marie, Ontario, to bring together research teams to integrate scientific knowledge on pests and forests in order to develop strategies and tactics for IPM. In the mid-1980s and into the 1990s, new monitoring systems and decision support systems (Shore and MacLean Reference Shore and McLean1997) were developed in an attempt to provide economic or action thresholds to guide control programmes. During this period, important advances were made in the development and application of pheromones for early pest detection, pest range determination, and measurement of the efficacy of pest management programmes (Evenden and Silk Reference Evenden and Silk2016).
Although several forest IPM programmes were initiated, forest pest managers quickly realised that the agricultural model was not wholly transferable to forestry applications. The methods used in agriculture for cost/benefit evaluation could not be easily applied in forestry because of difficulties in assessing pest damage owing to the reality that impacts of forest pests vary widely spatially and temporally, and are sometimes delayed and cryptic (Alfaro Reference Alfaro1988). A challenge for evaluation of the economic cost/benefit in forest pest management is the typical long temporal lag between the occurrence of the damage and time when we harvest the product, that is, timber. This causes the present net value of the benefit of control to be lower when the lag between damage and harvest is long (see Epanchin-Niell and Liebhold (Reference Epanchin-Niell and Liebhold2015) for an analysis of the economics of invasive pest prevention).
Despite the lack of hard economic data to define precise cost/benefit thresholds, many concepts of IPM were incorporated into the management of forest pests and helped guide management actions and IPM systems in Canada (Nealis Reference Nealis2015). These systems all favoured damage prevention or outbreak risk reduction as a starting point. Canadian researchers have investigated several options for outbreak risk reduction. For example, Whitehead and Russo (Reference Whitehead and Russo2005), proposed preventative thinning to enhance tree vigour and optimise the negative effects of microclimate on mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae) populations, as a method of outbreak prevention or “beetle-proofing”, and Fuentealba and Bauce (Reference Fuentealba and Bauce2012) recommended reducing spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), damage by carefully selecting plantation sites where soil drainage is less favourable to insect development, as they believed that this technique enhanced the mechanisms of resistance of trees.
Alfaro et al. (Reference Alfaro, Borden, Fraser and Yanchuk1995) synthesised knowledge of the natural history, damage, and control of the white pine weevil, Pissodes strobi (Peck) (Coleoptera: Curculionidae), a pest of spruce, Picea Dietrich (Pinaceae), and pines, Pinus Linnaeus (Pinaceae), in North America, to formulate an IPM system to reduce damage by this weevil. Significant progress was made in the area of tree genetic resistance to this weevil, and stock with different levels of resistance is now available in British Columbia (Alfaro et al. Reference Alfaro, King and van Akker2013), which provided a new tool for reducing plantation damage. The system relies on damage prevention by accurate hazard rating of plantation sites before planting (Hodgkinson et al. Reference Hodgkinson, White and Stock2011), and requires continuous monitoring of attack levels after planting. This IPM system recommends silviculture-driven tactics, such as shade conservation and increased plantation density, in low weevil hazard areas and resistance-driven tactics in high hazard areas. Decisions regarding which IPM tactics to employ are based on forecasting plantation productivity under various pest-level scenarious through the use of a decision support system (Alfaro et al. Reference Alfaro, Brown, Mitchell, Polsson and McDonald1997; Cruickshank et al. Reference Cruickshank, Sturrock, Di Lucca and Alfaro2015).
Shepherd and Otvos (Reference Shepherd and Otvos1986) developed an IPM system for the Douglas-fir tussock moth, Orgyia pseudotsugata McDunnough (Lepidoptera: Lymantriidae), in British Columbia, which was revised by Maclauchlan et al. (Reference Maclauchlan, Hall, Otvos and Brooks2009). This programme relies on pheromone trapping to determine adult concentrations, close monitoring of egg densities, and microbial control using nuclear polyhedrosis virus (NPV), when needed. In this manner localised, incipient outbreak populations of tussock moth could be detected before significant defoliation, and NPV could then be applied only where needed. The application of NPV to sites with increasing tussock moth populations effectively terminated localised infestations.
Two of the spruce budworm species are among most damaging defoliating insects of North America as outbreaks can be frequent, cover large areas, and cause significant growth loss and mortality of trees. The spruce budworm defoliates spruce and balsam fir, Abies balsamea (Linnaeus) Miller (Pinaceae), throughout the boreal forests of Canada, and the western spruce budworm, Choristoneura freemani Razowski (formerly C. occidentalis Freeman) (Lepidoptera: Tortricidae), is damaging to Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco, (Pinaceae), various species of fir, Abies Miller (Pinaceae), and spruce in western North America.
The IPM approach to spruce budworm and western spruce budworm are similar (Hopkin et al. Reference Hopkin, De Groot, Pitt, Carroll, Ottens and Caldwell2003; Carter Reference Carter2005) and usually involves aerial spraying of a biological insecticide, Bacillus thuringiensis Berliner (Bacillaceae) (Bt), when required. Spray programmes are supported by extensive monitoring to delineate the area of forest affected, and by population forecasts using weather-based models (Régnière et al. Reference Régnière, St-Amant and Béchard2013). When monitoring shows that populations are increasing, Bt is applied to areas considered at risk. The development of a computer-based decision support system for the spruce budworm (see MacLean Reference MacLean2016) allows for the integration of monitoring with knowledge of stand vulnerability and potential timber impacts so that losses can be reduced by early removal of forests at risk and the benefits of spraying selected stands can be maximised.
Although forest IPM had remarkable successes in guiding forest managers in their decisions when faced by threats, like the current spruce budworm outbreak in eastern Canada, the potential range expansion of the mountain pine beetle in British Columbia and Alberta, or the new threats posed by invasive species such as the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), the complexities and large spatial scale of forest ecosystems posed huge challenges for the development and full implementation of successful IPM systems in Canadian forests. Also, new ideas emerged, such as the concepts of forest health, sustainable forest management (SFM) and ecosystem management (EM), which forced a revision of forest IPM principles, as these were found to be pest-centric, and did not consider forest ecosystems as a whole.
Emergence of the sustainable forest management concept
The ways in which forests are perceived, conserved, and used has changed dramatically in the last 25 years. Forests are no longer seen simply as a source of fibre, but rather as complex ecosystems that sustain livelihoods and provide a range of products, values, and environmental services (e.g., carbon sequestration, water regulation, and biodiversity maintenance). These new views on the roles and values of forests emerged due to increasing international and national societal pressures for more diverse and equitable socio-economic development, reduced environmental impacts of industrial development, and assurance that a full suite of forest values will be sustained for present and future human generations. In other words, there was a desire to promote sustainable social development through the pursuit of national interests, based on holistic collective agreements by all stakeholders (Mery et al. Reference Mery, Alfaro, Kanninen and Lobovikov2005). The Brundtland Commission report in 1987 (World Commission on Environment and Development 1987) and the subsequent Rio Convention on Biological Diversity in 1992 (Convention on Biological Diversity 2016) set the stage for the implementation of sustainable development throughout the world.
Within the forest sector, a new paradigm emerged, SFM, often referred to as an EM approach to forest management (Mery et al. Reference Mery, Alfaro, Kanninen and Lobovikov2005). This paradigm shift was triggered by a profound change in how forest ecosystems were perceived and valued by society, which demanded at various local and international fora the need for SFM. News media and non-governmental organisations have played an important role in rapidly spreading, often through social networks, the negative news about the declining state of world forests, especially the world-wide alarming progress of deforestation. Deforestation in many places around the world has resulted in biodiversity loss, alteration of nutrient cycles and reduction of the capability of forests to sequester carbon, a key factor in combating global warming. In Canada, serious controversy regarding forest management practices began about 25 years ago (e.g., Pratt and Urquhart Reference Pratt and Urquhart1994) as these practices, or their effects on sustained timber and non-timber values were not seen to be sustainable. Thus, in Canada and globally, policy makers and the forest industry were pressured to adopt urgent agreements, plans, programmes, and actions for developing SFM practices.
The new paradigm of SFM-EM appeared first in the United States of America in the 1990s, replacing the traditional multiple-use forest management doctrine, as a new ideology, philosophy, policy, and practice to achieve SFM (Committee on Forestry Research 1990). This ecological approach attempts to meet the needs of the population while conserving biodiversity and the non-timber values of the ecosystems; that is, it is an approach to balance the economic, ecological, and social values of forests (Galindo-Leal and Bunnell Reference Galindo-Leal and Bunnell1995; Kohm and Franklin Reference Kohm and Franklin1997; Bergeron et al. Reference Bergeron, Harvey, Leduc and Gauthier1999). This paradigm shift occurred after forest ecologists and forest professionals realised that ecosystems are so complicated that the existing sustained yield, multiple-use approach did not guarantee sustainable ecosystems. It was concluded that it was impossible to achieve sustainable ecosystems simply by limiting extraction of individual ecosystem products and services, and that it was impossible to maintain biodiversity by focussing on the needs of every species individually.
Forest entomology in the sustainable forest management era
Landscape ecology, a relatively new discipline (Turner et al. Reference Turner, Gardner and O’Neill2001), recognised that ecosystems are not islands, but are intertwined in a matrix that is dynamic and subjected to natural as well as human-made disturbances. Thus, the present mosaic of forest patches found across the landscape is the result of the recurrent and intertwined actions of human-made and natural disturbances, such as fire and insect outbreaks that have shaped the landscape over hundreds of years. Forests and their constituent biodiversity have evolved to adapt to natural disturbance regimes and the changing forest structures they create. Therefore, biodiversity and the array of structures (patch size, species composition, and the like) of virgin landscapes, before the massive industrial development of the 20th century, are considered to be the benchmark or baseline to which interventions under EM should be compared. Ecosystems are considered healthy if the various processes and disturbance regimes are operating within the historic range of variation (Attiwill Reference Attiwill1994; Fule et al. Reference Fule, Covington and Moore1997), and biodiversity has emerged to be a surrogate measure of ecosystem function (Lapointe et al. Reference Lapointe, Langor, Dabros, Pinno, Spence, Pyper and Hirsch2015).
Insect pests as forest disturbances
Wildfire and outbreaking forest insects, such as budworms and mountain pine beetle, are the main natural stand-replacing disturbances affecting forests in Canada, having an impact on forests at varying scales depending on the intensity of the disturbance. Disturbances can be defined as relatively discrete events in time and space that disrupt the successional development of a forest stand, ecosystem, or landscape, by affecting its population structure, changing resources, substrate availability, the physical environment, and the processes of regeneration and recovery (Attiwill Reference Attiwill1994). Natural disturbances leave biological legacies (Seidl et al. Reference Seidl, Rammer and Spies2014; Alfaro et al. Reference Alfaro, Van Akker and Hawkes2015), which are defined as the organisms, organic matter (e.g., dead wood), and biologically created patterns (e.g., age classes) and functions that persist from the pre-disturbance ecosystem and influence recovery processes in the post-disturbance ecosystem (Franklin et al. Reference Franklin, Lindenmayer, MacMahon, McKee, Magnusson and Perry2000). Legacies play important roles in ecosystem resilience, that is the capacity of a system to retain its function, structure, identity, and feedbacks, following disturbance (Holling and Gunderson Reference Holling and Gunderson2002), by influencing rate of forest recovery following disturbance. Surviving forest patches, with their constituent habitats and environment, serve as reservoirs of biodiversity, sources of regeneration propagules, and nutrients, and provide landscape connectivity (Franklin et al. Reference Franklin, Mitchell and Palik2007). Consequently, the amount and spatial distribution of legacies (spatial heterogeneity) play a pivotal role in determining forest succession and recovery following disturbance (Campbell et al. Reference Campbell, MacLean and Bergeron2008; Turner et al. Reference Turner, Donato and Romme2013). For example, remnant patches of surviving trees (also referred to as secondary structure) are important legacies that remain after MPB outbreaks (Alfaro et al. Reference Alfaro, Van Akker and Hawkes2015; Campbell and Antos Reference Campbell and Antos2015) and wildfire. Legacies have the potential to act as a seed source for regeneration, increase structural complexity of canopies, speed up successional development and ecological recovery, and enhance the recovery of carbon stocks after disturbance (Gandhi et al. Reference Gandhi, Spence, Langor and Morgantini2001). Similarly, forest fragments in harvested landscapes provide reservoirs for species characteristic of interior forest habitats (e.g., Lee et al. Reference Lee, Spence, Langor and Pinzon2015).
A concept that clearly reflects the paradigm shift towards EM is the “emulation of natural disturbance” approach to management of forests (e.g., Hunter Reference Hunter1993). Canadian projects such as the Ecosystem Management Emulating Natural Disturbances (EMEND) (Spence Reference Spence2001; EMEND 2008) explore how variable retention harvesting approaches that better imitate the way natural processes, such as wildfire, operate. The incorporation of dispersed and aggregated retention in harvested stands clearly provides benefits for conservation of biodiversity on landscapes (Lee et al. Reference Lee, Spence, Langor and Pinzon2015). There is still much forest entomology work to be done in developing forest management methods that emulate natural disturbances other than fire, such as outbreaks of forest pests, which may be more prevalent than wildfire in some forest types and regions of Canada.
Further development of concepts about the sustainability of forests, articulated in the Montreal Process and the Santiago Declaration in 1995, culminated in the development of a set of ecological and socio-economic criteria and indicators to objectively measure sustainability (Canadian Council of Forest Ministers 2006). Under “Criterion 2: maintenance and enhancement of forest ecosystem condition and productivity”, a core indicator that addressed insects as disturbance agents is “Area and severity of insect attack”. These critera and indicators are now embedded in the latest version of the United Nations sustainable development goal 15: to sustainably manage forests, combat desertification, halt and reverse land degradation, halt biodiversity loss (United Nations 2016). In this context, Canadian forest entomologists are being compelled to consider if insect outbreaks negatively affect forest sustainability or if they are part of healthy natural ecosystems. Landscape ecologists have developed the concept of natural range of variation, which is defined as the long-term state of ecosystem characteristics and processes that prevailed before the large scale European settlement in North America (Government of British Columbia 2015). The natural range of variability refers to the full range of ecosystem structures, such as forest patch size, and processes, such as size and intensity of periodic insect or fire episodes. Thus, in order to assess the ecological relevance of insect disturbance to forest ecosystem sustainability, entomologists need to develop better understanding of the “natural” conditions of the forest landscapes, that is, before the massive antropogenic disturbance triggered by resource exploitation starting in the 20th century. Comparison of forest transformation by pests to the natural transformation cycles that occurred before the 20th century indicates if a present disturbance is operating outside the historic range, in terms of intensity and frequency, and will allow us to determine its ecological impact and whether interventions should be made. Some researchers have used the science of dendrochronology to determine the historical frequency of pest outbreaks in order to develop historic baselines of insect disturbance frequency and intensity. For example, extensive dendroentomology work has been completed to reconstruct the long-term history of C. fumiferana in the boreal forest of eastern Canada (e.g., Boulanger et al. Reference Boulanger, Arseneault, Morin, Jardon, Bertrand and Dagneaud2012). Alfaro et al. (Reference Alfaro, Berg and Axelson2014) reconstructed the history of western spruce budworm for the last 400 years in central British Columbia and calculated a return interval that averaged 28 years, with mean outbreak duration of nine years (Fig. 1). Under the EM paradigm, it can thus be inferred that forests are ecologically healthy when budworm outbreaks are recurring with that frequency and intensity.
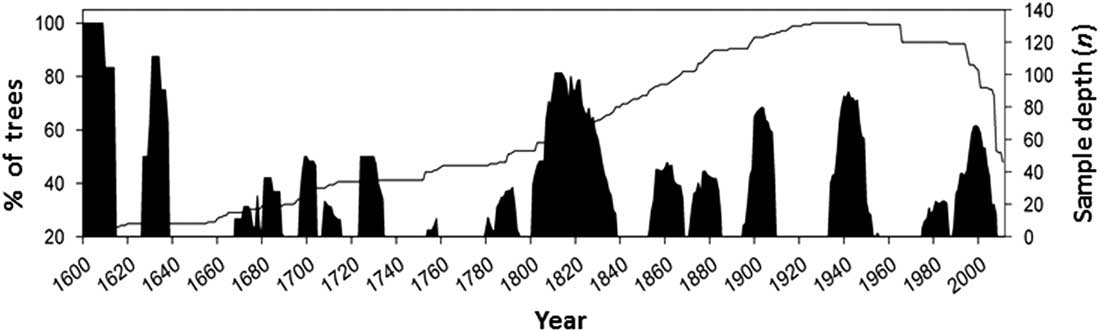
Fig. 1 History of western spruce budworm in central British Columbia for the last 400 years. Black columns indicate the percentage of Douglas-fir trees recording growth reductions attributed to western spruce budworm defoliation through time. The solid line indicates the sample depth (number of trees studied for growth reduction) (from Alfaro et al. Reference Alfaro, Berg and Axelson2014).
Insects as bioindicators and conservation targets
The majority of documented species in Canadian forests are insects and close relatives. The vast majority of the collective historical effort and investment in forest entomology in Canada has been focussed on the <1% of species that are considered pests. The principles of SFM-EM require consideration of the fact that all species contribute to ecosystem structures and functions, often in ways that are poorly understood and cannot be adequately measured. A wide diversity of species contributes to the sustainable flow of goods and services that are valued by society by maintaining normal functioning of ecosystems and resilience of forests to disturbance. Species – insects and others – are thus now valued as components of healthy and sustainable forest ecosystems, and conservation of biodiversity is a criterion of SFM (Canadian Council of Forest Ministers 2006).
The earliest study that called attention to the impacts of forest management, specifically harvesting by clear cutting, on insects in Canada was conducted from 1989–1991 (Niemelä et al. Reference Niemelä, Langor and Spence1993). Since then there has been a proliferation of entomological studies examining the impacts and mitigation of forest management that contribute to the evolution of EM. Concurrently, there has been an explosion of community ecology work on forest insect assemblages that seek to understand the patterns and determinants of biodiversity in unmanaged forests to develop the foundational understanding to interpret responses to forest management and other anthropogenic disturbances. Thus, entomological studies are major components of most modern forest management experiments in Canada, for example, EMEND (2008) and Sylviculture et Aménagement Forestier Écosystémiques (SAFE 2016). Among the most commonly studied groups are epigaeic beetles (Coleoptera: Carabidae, Staphylinidae), saproxylic beetles (Coleoptera: Staphylinidae), ants (Hymenoptera: Formicidae), and Lepidoptera. These groups of insects have proven to be useful bioindicators of ecosystem disturbance and recovery as they are abundant, easily sampled, taxonomically and trophically diverse, and readily identified to species.
Forest entomology in the 21st century
As indicated above, it is now recognised that most native insects are not “pests” as described in the old paradigms, but are rather integral parts of well-functioning ecosystems that need to be managed and conserved. Thus, there has been a dramatic shift in forest entomology over the last quarter of the 20th century, from a near-sole focus on insects as agents of destruction to one that balances ecological disturbance issues with recognition that insects are an integral part of healthy natural ecosystems. This is a trend that has continued into the early part of the 21st century. It has become increasingly evident that the best approach to ensuring that insects (and the ecological services they provide) continue to be a part of healthy forest ecosystems, and that species prone to negatively impacting other forest values (e.g., fibre) are kept in check, is ensuring that management is grounded in science. The goals of this science, and the challenges for forest entomologists, are to understand, among other topics, the ecological roles of species; what factors determine species distribution and abundance; and the impacts of natural and anthropogenic disturbances on species, assemblages and functions. This information could then be used to develop strategies that reduce the risk of insect disturbance affecting the flow of forest goods and services. We highlight four broad areas of entomological endeavour that would benefit from significant new science investment and best help understand entomological components of forests.
-
1. Study of the endemic (non-outbreak) phase of population cycles. Insect species that are prone to catastrophic outbreaks that cause significant damage to timber resources have been the subject of much research; however, the availability of research resources tends to closely follow the insect population curve. Thus, endemic phases of population cycles have received little research attention, despite the fact that this is the longest lasting phase of cycles and is the phase in which species populations switch from low levels to one with rapid growth. Thus the processes that lead to this switch remain poorly understood for almost all significant forest pests in Canada. Work to understand the ecological interactions with hosts, competitors, natural enemies, and abiotic components of the environment during the endemic phase is critical to understanding why outbreaks occur, and ultimately for development of preventative pest management measures. As well, for many outbreaking species, there is not yet clarity on why populations collapse.
-
2. Cumulative environmental impacts and restoration ecology. Canada’s forest ecosystems are under increasing pressure from development, and the variety of types of disturbances is increasing on some landscapes, for example, in situ oil sands development is rapidly expanding in the western boreal forest. While insects have been used as bioindicators over the last 30 years to assess the impacts of natural and anthropogenic disturbances, typically disturbances have been examined in isolation, focussing mainly on harvesting and wildfire, to measure impacts. However, the fact is that it is increasingly rare that forest stands and landscapes are subjected to just one disturbance but are rather experiencing the cumulative impacts of multiple disturbances that are natural (e.g., wildfire, insect outbreaks) and anthropogenic disturbances (harvesting, conventional oil and gas, conventional mining, oil sands mining and in situ development, agriculture, transportation, and communication corridors). Each disturbance type creates a unique spatial pattern and chemical/geophysical impacts what lead to different biological impacts. To understand and predict the impact of human activities on forest landscape, it is essential to understand how multiple natural and anthropogenic disturbances interact and how impacts accumulate. To date, this has rarely been the subject of entomological attention (but see Cobb et al. Reference Cobb, Langor and Spence2006). As well, there is increasing need to develop ecologically sound sustainability indicators and restoration approaches for industrially developed sites and landscapes. To date, there has been little entomological involvement in addressing these challenges.
-
3. Climate change influences. As climates in Canada are already changing rapidly, and will continue to change, the pervasive impacts of such change affect practically all aspects of terrestrial organism natural history. Climate change will result in longer growing seasons, extreme temperatures, variable precipitation conditions, altered wildfire regimes, changes in plant distributions. For native insects, such changes will result in responses such as: spread of species into novel ecosystems, extirpation and possible extinction of species from areas no longer with climatically suitable habitats, altered population cycles with resulting impacts on host health and survival, and altered interactions with other species. The opportunities for entomological work to document ongoing changes (impacts and adaptations) and predict trends are enormous. The interaction between climate change and other anthropogenic and natural disturbances is largely unexplored. Although there is clearly a need for entomological work, the enormous challenges posed by climate change and cumulative impacts require highly integrated multidisciplinary approaches.
-
4. Non-native species. Non-native insect species have colonised and continue to spread in most Canadian ecosystems (Langor et al. Reference Langor, DeHaas and Foottit2008, Reference Langor, Cameron, MacQuarrie, McBeath, McClay and Peter2014). The increasing industrial development and changing climates will provide continued opportunities for establishment and spread of non-native species, including in more remote areas. While there has been considerable attention paid to a small number of invasive insect species that have negatively affected commercially important tree species, the vast majority of non-native species do not manifest impacts in such conspicuous ways. Thus, an important area of research that has received little attention is the study of the resistance of forest ecosystems to the establishment of non-native insects. Related to this is the fact that, for the large majority of species introduced into this country and now established, their ecological effects have not been documented. As many species have affinity for disturbed ecosystems, the cumulative changes caused by industrial development and climate change may make it easier for establishment of new non-native species, spread within Canada, and invasion of natural ecosystems, ultimately resulting in unprecedented ecological and economic impacts.
Conclusions
The science of forest entomology in Canada has sustained several abrupt changes or paradigm shifts in the last 100 years, moving from the initial descriptive stages, to a pest control era, to the IPM period and now to pest management as part of EM. Managing the landscape for pests requires complex forest management systems capable of reflecting the emerging vision of EM. Such systems need to accommodate the analysis and projection of a range of values and ecological processes, including society’s sometimes-antagonistic need for forest products, biodiversity, wildlife habitat, and other values. The systems require that land managers understand the ecological processes that operate over large spatial and temporal scales. Fortunately, the recorded history of research by Canadian forest entomologists before us, summarised in this special issue, provides an initial information base for practicing pest management within the framework of EM.
Acknowledgement
The authors thanks Elizabeth Campbell for reviewing an early draft of this document.





