INTRODUCTION
In their natural habitat plants are exposed to a wide array of environmental factors. Being sessile in nature, plants are forced to adapt to various environmental stresses, which causes a wide range of alterations at cellular and molecular level. This in turn reduces production and productivity of plants (Shao et al. Reference Shao, Chu, Jaleel and Zhao2008). Survival and successful reproduction in a stressful environment is a complex phenomenon and decisive for food security from the perspective of climate change. It is synchronized and regulated by physiological, cellular and molecular activities of plants (Ahuja et al. Reference Ahuja, de Vos, Bones and Hall2010). Plant survival in stressful environments has a physiological cost (Massad et al. Reference Massad, Dyer and Vega2012), which becomes a major constraint for growth and development and can reduce yield by >50% in major crops (Bray et al. Reference Bray, Bailey-Serres, Weretilnyk, Gruissem, Buchannan and Jones2000). Frequent fluctuations in temperature and uncertain precipitation limit productivity and cause loss of diversity. Indian agriculture is highly dependent on the spatial and temporal distribution of monsoon rainfall (Kumar et al. Reference Kumar, Kumar, Ashrit, Deshpande and Hansen2004). As per future projections, heavy rains or drought are equally probable in the future (Allen & Ingram Reference Allen and Ingram2002) and this will increase the range as well as intensity of various stresses. In order to maintain sustainable agriculture under these conditions, there is a strong need to develop new alternatives (Khush Reference Khush1999) such as exploration and identification of diverse germplasm with known traits, which can provide a practical solution to alleviate the problem of water limitation. In this context exploration, collection and evaluation of untapped diversity from different ecological regions is of paramount importance.
Early perception of stress signals by plants and immediate response is important for environmental stress tolerance. After recognition of stress signals, basal mechanisms operative within plants (Andreasson & Ellis Reference Andreasson and Ellis2010) lead to an activation of complex signalling cascades of tolerance, varying from one stress to another (Abu Qamar et al. Reference Abu Qamar, Luo, Laluk, Mickelbart and Mengiste2009). In most cases, experiments designed for stress tolerance consider plant responses to individual stress (Qin et al. Reference Qin, Shinozaki and Yamaguchi-Shinozaki2011; Todaka et al. Reference Todaka, Nakashima, Shinozaki and Yamaguchi-Shinozaki2012); however, the response to multiple stresses is much more complex (Fujita et al. Reference Fujita, Fujita, Noutoshi, Takahashi, Narusaka, Yamaguchi-Shinozaki and Shinozaki2006). Although, research on multiple stresses has been trying to simulate natural conditions, however in the field, conditions are not manually controlled and Space bar should be used between one stress can strongly influence the primary stress defence response of plants (Fujita et al. Reference Fujita, Fujita, Noutoshi, Takahashi, Narusaka, Yamaguchi-Shinozaki and Shinozaki2006).
As a consequence of exposure to environmental stress, reactive oxygen species (ROS) increase in cells (Laloi et al. Reference Laloi, Appel and Danon2004; Foyer & Noctor Reference Foyer and Noctor2005) and leads to reprogramming of gene expression resulting in an increase in plant tolerance. This also minimizes the biological damage caused by the stress (Fujita et al. Reference Fujita, Fujita, Noutoshi, Takahashi, Narusaka, Yamaguchi-Shinozaki and Shinozaki2006). In plants grown in stressful environments, ROS have been perceived as destructive and harmful compounds. Although low levels are mostly responsible for regulating plant stress responses, high levels of ROS lead to cell death (Choudhury et al. Reference Choudhury, Panda, Sahoo and Panda2013). Therefore, the ROS status of plants, and their enzymic and non-enzymic defence mechanism in relation to response to environmental stresses may be utilized to screen germplasm for environmental stress tolerance.
‘Millet’ is a collective term used to refer to a diverse group of small-seeded annual C4 Panicoid grasses such as barnyard millet (Echinochloa frumentacea), finger millet (Eleusine coracana), foxtail millet (Setaria italica) and proso millet (Panicum miliaceum). These are cultivated as food and fodder crops in temperate, sub-tropical and tropical regions across the globe (Dwivedi et al. Reference Dwivedi, Upadhyaya, Senthilvel, Hash, Fukunaga, Diao, Santra, Baltensperger, Prasad and Janick2012; Lata et al. Reference Lata, Gupta and Prasad2013) and have remarkable nutritional properties. Barnyard millet (Echinochloa spp.), also known as billion-dollar grass, madira, jhangora or sawan, is the second most important millet crop after finger millet, both in terms of acreage and production in the Central Himalayan Region (CHR). In India, the area under small millets has been steadily decreasing during the last three decades (FAO 2014) and in recent years the pace of decline has been much faster (Joshi Reference Joshi2013). Millet cultivation areas have shrunk nearly 42% over the last 50 years between 1956 and 2006; all millet growing areas in India have moved towards other crops (Sateesh Reference Sateesh2010). There are several factors responsible for this decline: the availability of alternative crops with greater market value, such as rice and pulse crops, as well as lack of government support may be attributed as main reasons. In CHR, barnyard millet is a mainstay of the diet and cultural systems of hill people (Kumar et al. Reference Kumar, Kumar and Yadav2007). It is the fastest growing crop among all millets and can be harvested in a short period of 9 weeks. The crop is known for its good yield and high nutritional value (Prabha et al. Reference Prabha, Negi and Khanna2010). Despite its significance, barnyard millet has largely been an under-researched crop compared with the main staple cereals. Millets are considered as minor cereal crops of only regional importance; hence little attention has been given to collection, conservation and evaluation of available diversity for use in crop improvement. Since millets are grown in low-input, rain-fed agricultural systems, they tend to suffer from a range of environmental stresses that become major constraints for crop production and yield. The area under study in the current work is prone to vagaries of weather, i.e. frequent fluctuation in temperature, erratic rainfall, drought, hailstorms, etc. Therefore, evaluation of whole germplasm in the field may be a practical strategy to screen trait-specific germplasm for crop improvement and climate resilience as well as cultivation in this and other such agro-ecological areas.
MATERIALS AND METHODS
Morpho-physiological traits
In total 178 accessions of barnyard millet having unique traits of agronomic importance were collected from altitudinal range of 175–2250 m a.s.l. in the CHR (Fig. 1) and evaluated in the field under rain-fed conditions at an experimental site located at 29°24′ N, 79°30′ E, 1480 m a.s.l. Experiments were conducted during the Kharif season (June–October) for three consecutive years, i.e. 2011–2013, in an augmented block design (ABD). Five representative plants of each accession were tagged in each block for recording observations. Data for various morphological traits were recorded following the procedure described by Trivedi et al. (Reference Trivedi, Arya, Verma, Verma, Tyagi and Hemantaranjan2015).
Fig. 1. Map of Central Himalayan Region and experimental site.
Biochemical analysis
Fresh leaf tissues taken at the flowering stage were extracted in 80% acetone for spectroscopic estimation of chlorophyll (Strain et al. Reference Strain, Bengamin, Walter and Pietro1971) and carotenoid content (Duxbury & Yentsch Reference Duxbury and Yentsch1956). Lipid peroxidation was measured by the thiobarbituric acid test as described by Dhindsa & Matowe (Reference Dhindsa and Matowe1981). The methods described by Bostock et al. (Reference Bostock, Yamamoto, Choi, Ricker and Ward1992) and Cordewener et al. (Reference Cordewener, Booij, van der Zandt, van Engelen, van Kammen and de Vries1991) were used for measuring lipoxygenase (EC 1·13·11·12) and peroxidase (EC 1·11·1·7) activity, respectively. Catalase (EC 1·11·1·6) and superoxide dismutase (SOD) (EC 1·15·1·1) activity was determined by the methods described by Rao et al. (Reference Rao, Paliyath and Ormrod1996) and Beauchamp & Fridovich (Reference Beauchamp and Fridovich1971), respectively. The method of Wang & Luo (Reference Wang and Luo1990) was followed for determination of superoxide radical (O2 −.) generation rate. Glutathione contents [reduced glutathione (GSH) and oxidized glutathione (GSSG)] were determined enzymatically using the method of Griffith (Reference Griffith1980). Glutathione reductase (GR) (EC 1·6·4·2) was measured following Smith et al. (Reference Smith, Vierheller and Thorne1988). Both reduced (AsA) and oxidized (DAsA) ascorbate content were determined as described by Knörzer et al. (Reference Knörzer, Durner and Boger1996), adapted from the bipyridyl method of Masato (Reference Masato1980). Monodehydroascorbate reductase (MDHAR) (EC 1·6·5·4) and dehydroascorbate reductase (DHAR) (EC 1·8·5·1) activity were assayed according to the method of Hossain et al. (Reference Hossain, Nakano and Asada1984) and Hossain & Asada (Reference Hossain and Asada1984), respectively. The activity of ascorbate peroxidase (APX, EC 1·11·1·11) was assayed by the method of Nakano & Asada (Reference Nakano and Asada1987).
Statistical analysis
The statistical analysis for principal component, clustering graph (Ward Reference Ward1963) and k-means clustering was performed using statistical software SAS 9·3. Principal component analysis (PCA) was done to identify a smaller number of uncorrelated variables to explain the maximum amount of variance with the fewest number of principal components. Clustering was done to partition groups of data points into a small number of clusters.
RESULTS
Flag leaf length of germplasm collected from CHR was found to vary from 130·0 mm in IC282785 to 353·4 mm in IC261999. Similarly, flag leaf width varied from 10·0 mm in IC282785 to 47·2 mm in IC355786. Peduncle length ranged from 20·0 mm in IC282785 to 124·0 mm in IC469750. Approximately three-fold variability was found in plant height, which ranged from 546·6 mm in IC279563 to 1612·3 mm in IC273988. Ear length, which is directly related to seed production, was found to vary from 111·5 mm in IC337349 to 237·2 mm in IC382642. Days to 50% flowering and days to 80% maturity, crucial parameters related to the life span of a crop, were found to vary from 48·12 in IC279535 to 88·00 in IC279391 and from 96·00 in IC273927 to 132·66 in IC317641, respectively. Thousand seed weight ranged from 2·03 g in IC355791 to 5·80 g in IC261959. Similarly, yield per plant was found to vary from 0·25 g in IC279408 to 9·25 g in IC273927 (Table 1).
Table 1. Variability in morphological traits, flowering and yield of barnyard millet (Echinochloa frumentacea) accessions of Central Himalayan Region

* Accession numbers having maximum or minimum value are given within parenthesis in the respective columns on the right side of the value.
Chlorophyll content at the flowering stage was found to vary from 1·024 mg/g FW (fresh weight (FW)) in IC418409 to 6·859 mg/g FW in IC548696. Similarly, carotenoid content at the flowering stage ranged from 0·563 mg/g FW in IC261951 to 6·325 mg/g FW in IC337304. Lipid peroxidation, the most easily ascribed symptom of membrane damage, ranged from 0·552 nmol malondialdehyde (MDA) formed/mg protein/h in IC391472 to 7·421 nmol MDA formed/mg protein/h in IC338652. Lipoxynase activity ranged from 0·124 mmol substrate/min/mg protein in IC282785 to 4·023 mmol substrate/min/mg protein in IC279391. Activity of catalase, which splits toxic hydrogen peroxide into oxygen and water, was found to vary from 109·00 mmol hydrogen peroxide decomposed/min/mg protein in IC261951 to 855·00 mmol hydrogen peroxide decomposed/min/mg protein in IC340999. Activity of peroxidases, which are involved in many physiological processes in plants including responses to abiotic stresses, was found to vary from 1·236 mmol substrate/min/mg protein in IC281760 to 6·355 mmol substrate/min/mg protein in IC548613. Superoxide dismutase activity, which is known to control oxidative stress in plants, was found to vary from 1123·00 enzyme U/mg protein in IC261951 to 2963·00 enzyme U/mg protein in IC279703. Superoxide free radical (a toxic compound) was found to range from 0·452 nmol hydrogen peroxide formed/mg protein in IC261951 to 4·285 nmol hydrogen peroxide formed/mg protein in IC469893.
The lowest contents of total, reduced and oxidized glutathione (105·270, 96·217 and 9·180 mmol/g FW, respectively), as well as the lowest level of glutathione reductase activity (0·532 mmol substrate/min/mg protein) were all found in IC355792. Similarly, IC355775 had maximum total, reduced and oxidized glutathione content (423·630, 387·20 and 36·941 mmol/g FW, respectively) as well as glutathione reductase activity (2·139 mmol substrate/min/mg protein).
The least amounts of total ascorbate (4·980 mmol/g FW), ascorbic acid (4·235 mmol/g FW) and dehydroascorbic acid (0·676 mmol/g FW) were found in IC261971, IC548641 and IC418409, respectively. The highest amounts of total ascorbate (9·880 mmol/g FW) and dehydroascorbic acid (1·657 mmol/g FW) were found in IC355769, whereas the highest amount of ascorbic acid (8·600 mmol/g FW) was found in IC393054. The activity of ascorbate peroxidase, which detoxifies peroxides such as hydrogen peroxide using ascorbate as a substrate, was found to vary from 1·860 enzyme U/mg protein in IC355803 to 7·040 enzyme U/mg protein in IC355796. Monodehydroascorbate reductase activity varied from 1·106 mmol substrate/min/mg protein in IC391472 to 4·41 mmol substrate/min/mg protein in IC355796. Dehydroascorbate reductase activity varied from 0·330 mmol substrate/min/mg protein in IC261951 to 1·359 mmol substrate/min/mg protein in IC355796 (Table 2).
Table 2. Variability in biochemical traits of barnyard millet (Echinochloa frumentacea) genepool of Central Himalayan Region
MDA, malondialdehyde.
* Accession numbers having maximum or minimum value are given within parenthesis in the respective columns on the right side of the value.
Cluster analysis was done by Ward's method to divide observations into homogeneous and distinct groups, in which two main clusters were formed. These clusters were further divided into seven sub-clusters. Hence, K means clustering was done to divide all 178 accessions into seven clusters (Table 3). Accessions with similar traits were found to group together. Three accessions grouped in Cluster 1 have very close similarity in morpho-physiological traits. Cluster 7 has the maximum number of accessions, i.e. 48; these accessions all show close similarity in morpho-physiological traits within the cluster. Accessions within a cluster may be useful for selection of similar genotypes for a particular trait, as well as for breeding programmes.
Table 3. K-means clustering of germplasm

It is evident from PCA and percentage contribution of each component to the total variation (Table 4) that the first four variables contributed 99·94% of the variability (55·24, 38·24, 5·10 and 1·36% of the total variability from the first, second, third and fourth principal components, respectively). It is obvious from the scree plot of the principal components (Fig. 2) that only four principal components contribute considerably towards diversity.
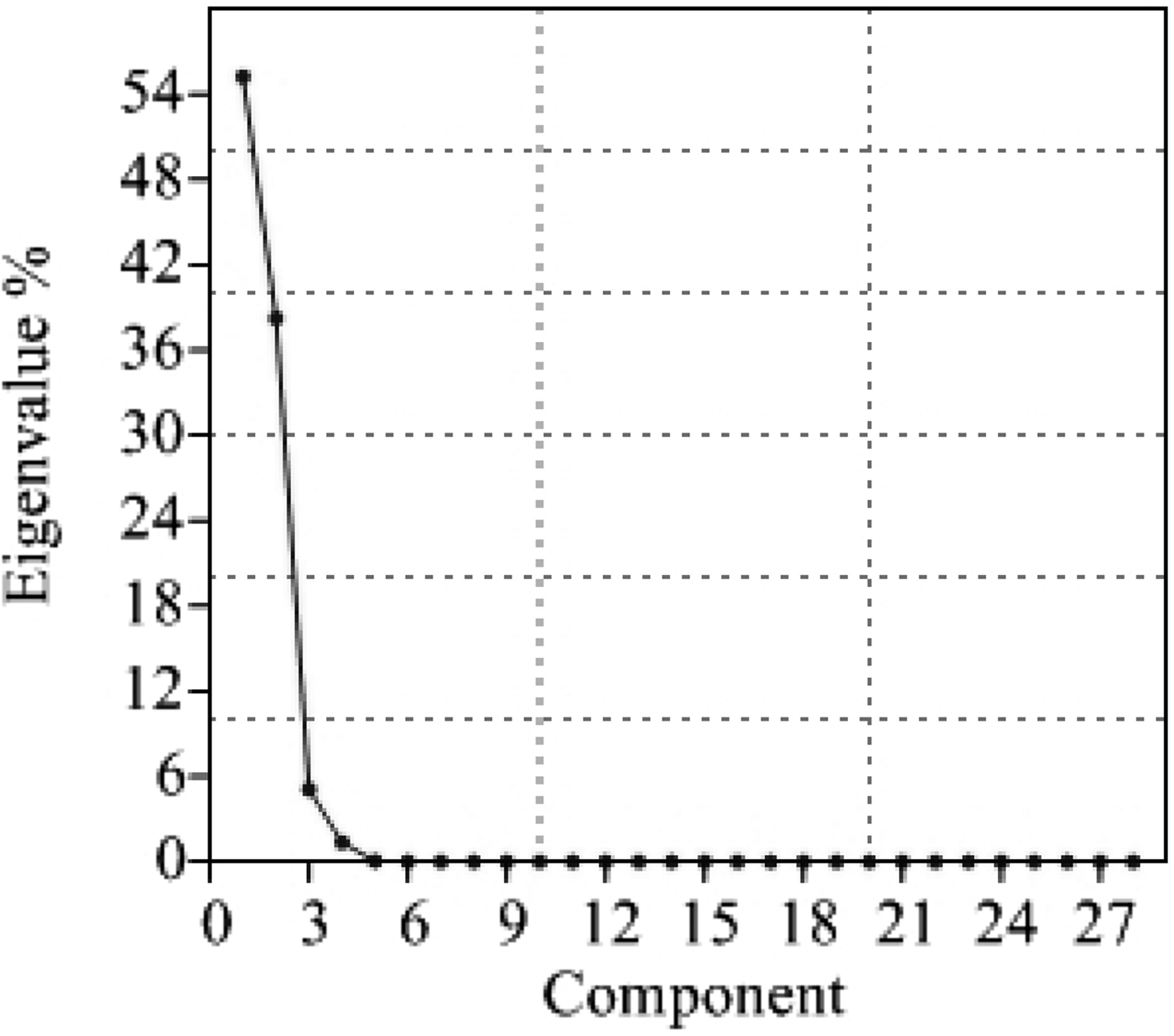
Fig. 2. Scree plot of principal components.
Table 4. Contribution of the first four principal component axes to variation in barnyard millet (Echinochloa frumentacea) based on morpho-physiological and biochemical traits

MDA, malondialdehyde.
DISCUSSION
The area under study is characterized by considerable diversity in millet types. However, uneven distribution of precipitation and abrupt changes in temperature upsets biochemical processes at cellular and molecular levels, which impedes normal life processes. This in turn affects plant growth and development; plants growing in adverse climatic conditions differ in shape and size compared with those growing in normal/favourable conditions. Vegetative growth under stress conditions, particularly shoot growth, decreases due to slower cell division and growth (Schuppler et al. Reference Schuppler, He, John and Munns1998). Significant variation in leaf dimensions in the present study indicates the variable ability of different accessions to adapt to the surrounding environment. This is supported by the studies of Sisó et al. (Reference Sisó, Camarero and Gil-Pelegrín2001) and Pandey & Nagar (Reference Pandey and Nagar2002), who suggested that modifications in leaf shape and size are early symptoms of plant adaptation to growth habitat. It also provides a link between various environmental factors and leaf functions (Roche et al. Reference Roche, Díaz-Burlinson and Gachet2004). In addition, plant height is an appropriate determinant of a plant's ability to compete for light (Falster & Westoby Reference Falster and Westoby2003), particularly in dense fields of cultivated crops. Plant height is also an important part of life-history traits (Moles & Leishman Reference Moles, Leishman, Leck, Parker and Simpson2008). Approximately three-fold variation in plant height of the germplasm studied here indicates rich diversity in this particular trait and the ability of plants to adjust to environmental conditions. In adverse environmental conditions, several pheno-morphological traits relate to the competitive ability of a crop to survive and yield optimally, such as plant height (Lindquist et al. Reference Lindquist, Mortensen and Johnson1998), leaf angle (Sankhala et al. Reference Sankhala, Chopra and Sankhala2004) and crop maturity (Begna et al. Reference Begna, Smith, Hamilton, Dwyer and Stewart2001). Substantial variability found in these traits helps the germplasm to survive and produce seeds in otherwise adverse agro-climatic conditions. These traits are central in determining how a species lives, grows and reproduces. Life-span as well as different pheno-phases of a crop is affected by surrounding conditions. In plants, switchover from the vegetative to reproductive phase is a crucial turning point, which is under the control of a complex genetic network that integrates information from various endogenous and environmental cues (Amasino Reference Amasino2010). Among the environmental factors that may affect plant growth, only a few appear to be specifically monitored to control flowering (Amasino Reference Amasino2010; Srikanth & Schmid Reference Srikanth and Schmid2011). The shift from vegetative to reproductive phase ensures that plants set their flowers at an optimum time for pollination, seed development and dispersal (Cockram et al. Reference Cockram, Jones, Leigh, O'Sullivan, Powell, Laurie and Greenland2007). Difference in flowering time of germplasm can be used to increase yield and extend agricultural flexibility as well as the eco-geographical range of crops (Cockram et al. Reference Cockram, Jones, Leigh, O'Sullivan, Powell, Laurie and Greenland2007). Noteworthy variation in days to 50% flowering and days to 80% maturity of germplasm was found in the current study. Collected germplasm has an inherent ability to grow and produce economic yield in environments entirely different from the site of collection. Considerable variability in yield attributes and yield seems to be due to the net result of direct and indirect effects of the component characters from which grain yield is derived (Prasanna et al. Reference Prasanna, Murthy, Ramakumar and Rao2013).
Furthermore, alteration in the activity of antioxidant enzymes is an adaptation to stress and a defence process. Significant variability in the lipid peroxidation of different accessions denotes the symptom most easily attributed to oxidative damage (Zhang & Kirkham Reference Zhang and Kirkham1996). It also indicates that plants experience oxidative stress (Jagtap & Bhargava Reference Jagtap and Bhargava1995), but adjust to survive and produce an economic yield. Variability in lipoxygenase activity may be due to variability in the hydroperoxidation of polyunsaturated fatty acids. Remarkably, eight-fold variability in catalase activity indicates considerable variability of germplasm to tolerate stress conditions. Catalase lowers oxidative damage by converting hydrogen peroxide to water and oxygen (Scandalios et al. Reference Scandalios, Guan, Polidoros and Scandalios1997). Up-regulation of the gene for catalase enzyme activity protects leaves against ROS (Zelitch et al. Reference Zelitch, Havir, McGonigle, McHale and Nelson1991); in contrast, catalase-deficient plants are more sensitive to various stresses (Willekens et al. Reference Willekens, Chamnongpol, Davey, Schraudner, Langebartels, Van Montagu, Inzé and Van Camp1997). Moreover, ascorbate peroxidase is a key enzyme in the ascorbate-glutathione cycle, the main hydrogen peroxide-detoxification system found in plant chloroplasts (Asada Reference Asada1992). Ascorbate peroxidase also plays a role in ROS scavenging in cytosol, mitochondria and peroxisomes (Noctor & Foyer Reference Noctor and Foyer1998; Asada Reference Asada1999; Shigeoka et al. Reference Shigeoka, Ishikawa, Tamoi, Miyagawa, Takeda, Yabuta and Yoshimura2002; Mittler et al. Reference Mittler, Vanderauwera, Gollery and Van Breusegem2004). In agreement with a previous report, significant variation in peroxidase activity was found in different accessions due to environmental stress conditions prevailing in CHR (Shigeoka et al. Reference Shigeoka, Ishikawa, Tamoi, Miyagawa, Takeda, Yabuta and Yoshimura2002). Up-regulation of the peroxidase activity confirms the major role played by these enzymes in defence mechanisms (Jouili et al. Reference Jouili, Bouazizi and El Ferjani2011). However, scavenging of superoxide by SOD is an important mechanism to cope with stress conditions (Bowler et al. Reference Bowler, Van Montagu and Inzé1992). A small quantity of superoxide free radical was also found which may be scavenged simultaneously by SOD. Besides, variability in the activity of antioxidant enzymes and variation in the antioxidant pool is a distinctive symptom of stress tolerance. Plants adjust antioxidant levels as an adaptation to stress and a defence process. Differences in total glutathione content of various accessions might be due to variability in the capacity of accessions to overcome environmental stress. The tripeptide glutathione (GSH), an antioxidant, exerts a number of functions in plants (Paranhos et al. Reference Paranhos, Fernández-Tárrago and Corchete1999). In spite of frequent fluctuations in environmental conditions and erratic rainfall, the collected germplasm was able to survive and produce economic yield. This might be due to the protection of cells against the toxic effects of free radicals and the ability to keep free-radical scavenging ascorbate in its reduced, i.e. active form, by glutathione (Zhang & Kirkham Reference Zhang and Kirkham1996). Significant variation in glutathione reductase (EC 1·6·4·2) activity indicates that it sustains the reduced status of glutathione via the ascorbate-glutathione pathway. The activity of glutathione reductase is crucial for stress tolerance because its substrate GSSG, as well as its product GSH, is important for several cellular functions, such as cell division (Rebhun et al. Reference Rebhun, Miller, Schnaitman, Nath and Mellon1976), amino acid transport through membranes (Meister Reference Meister1981) and regulation of enzymatic activity (Holmgren Reference Holmgren1979). Correspondingly, variability in the ascorbate and ascorbate-recycling enzymes, i.e. monodehydroascorbate reductase and dehydroascorbate reductase, was also found, which is in agreement with the previous findings of Knörzer et al. (Reference Knörzer, Durner and Boger1996). These enzymes help to maintain the redox pool of ascorbate and in turn improve stress tolerance (Kim et al. Reference Kim, Kim, Shin, Park, Park, Kim, Lee, Kang, Lee and Yoon2014). In accordance with earlier reports (Shigeoka et al. Reference Shigeoka, Ishikawa, Tamoi, Miyagawa, Takeda, Yabuta and Yoshimura2002; Trivedi et al. Reference Trivedi, Arya, Verma, Verma, Tyagi and Hemantaranjan2015), APX activity was found to increase in response to stress conditions in the field. Although all the accessions were grown at one experimental site, enormous variability was recorded in antioxidant pool size and activity of oxidative stress enzymes, which indicates the potential of accessions to cope with the stress conditions. Based on the clustering of whole germplasm into seven groups having similar traits, appropriate germplasm may be selected for cultivation in areas prone to environmental/abiotic stresses; also climate-compliant accessions may be identified for cultivation in different agro-climatic zones. Rapid perception of abiotic stresses by plants and appropriate estimation of phenological and biochemical adjustments in response to stress are critical to ensure future food security. Based on morpho-physiological evaluation, suitable accessions might be selected for developing climate-resilient varieties.
Authors are thankful to Director, ICAR – National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi for providing necessary facility and keen interest in the study. Authors are also thankful to Dr Achal Singh (ICAR-Central Institute for Subtropical Horticulture, Lucknow) for help in statistical analysis of the data.











