To the Editor—Extensive barrier precautions can prevent skin and mucous membrane contamination during the patient care. However, personal protective equipment (PPE) can be contaminated with body fluids and infectious virus after a patient care encounter.Reference Tomas, Kundrapu and Thota 1 , Reference Verbeek, Ijaz and Mischke 2 We read with interest the articles by Casanova et alReference Casanova, Teal and Sickbert-Bennett 3 and Kwon et al,Reference Kwon, Burnham and Reske 4 which reported a certain level of self-contamination with nonenveloped viruses and a much lower rate of self-contamination with enveloped viruses, with contamination limited to inner gloves. Recently, Casanova et alReference Casanova, Erukunuakpor and Kraft 5 and Mumma et alReference Mumma, Durso and Ferguson 6 (from the Centers for Disease Control and Prevention Epicenters Program, Division of Healthcare Quality Promotion, United States) further assessed the contamination of skin, gloves, and scrubs after doffing Ebola-level PPE. In these studies, they assessed self-contamination risks using 2 surrogate viruses, bacteriophages MS2 and Φ6, to represent nonenveloped and enveloped viruses, respectively. However, given that both MS2 and Φ6 are spherical bacteriophages and are much smaller than the filamentous Ebola virus, their adhesion capabilities on PPE are much different. Thus, the reported contamination rates after doffing Ebola-level PPE may be inaccurate.
Ebola virus is an enveloped RNA virus with a filamentous appearance and a uniform diameter of ~80 nm, but Ebola virus particles vary greatly in length. In general, the median particle length of Ebola viruses ranges from 974 to 1,086 nm.Reference Feldmann and Klenk 7 In contrast, bacteriophage Φ6 has a pleomorphic appearance and a uniform diameter of ~80 nm,Reference Katz, Alimova, Futerman, Katz, Wei and Gottlieb 8 which is almost 10 times shorter than the average length of an Ebola virus particle (Figure 1). In microbial fermentations, small increases in hyphal length (eg, the formation of pellets or clumps) can cause large increases in broth viscosity because filamentous bioparticles have higher adhesion forces than spherical bioparticles.Reference Papagianni 9 Thus, the adhesion capability of Φ6 on PPE may be much lower than that of Ebola virus. Thus, the bacteriophage Φ6 might not be an ideal surrogate virus.
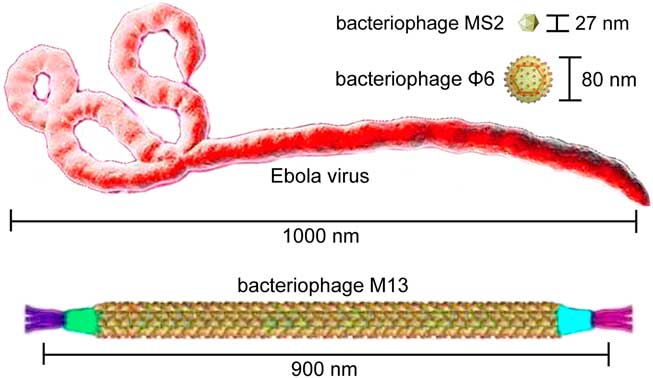
FIGURE 1 Lengths and shapes of Ebola virus and bacteriophages MS2, Φ6, and M13.
In detailed studies, Casanova et alReference Casanova, Erukunuakpor and Kraft 5 found that no Φ6 transfer to inner gloves, hands, or face among 10 healthcare workers. Only 1 healthcare worker had Φ6 on scrubs at low levels (1.4×102) This contamination rate was much lower than that of nonenveloped bacteriophage MS2: 2 healthcare worker had MS2 on scrubs, 1 had it on hands, and 7 had it on inner gloves (at 101–106). Despite these differences, the fault trees for MS2 and Φ6 contamination suggested similar pathways.Reference Mumma, Durso and Ferguson 6 Similarly, very low levels of Φ6 contamination (much lower than those of MS2) have also been reported in previous studies.Reference Casanova, Teal and Sickbert-Bennett 3 , Reference Kwon, Burnham and Reske 4 However, for the aforementioned reason, the risk the doffing protocol for Ebola-level PPE may be underestimated when Φ6 is selected as the surrogate virus. Also, the risk that the doffing protocol for Ebola-level PPE may be overestimated when MS2 is selected as the surrogate virus must be considered.
Different from Φ6 and MS2, M13 is a filamentous bacteriophage with a ~900 nm particle length,Reference Stopar, Spruijt, Wolfs and Hemminga 10 which is very close to the average length of Ebola viruses (Figure 1). The M13 bacteriophage can be easily cultured and detected with the visible fluorescent marker, making it an ideal surrogate virus for the biocontainment study on the Ebola-level PPE. Presumably, a more accurate contamination rate after doffing Ebola-level PPE could be achieved using the surrogate virus M13. Nevertheless, we agree with the authors that the doffing protocols should be improved for better protections against all types of viruses, especially the filamentous Ebola viruses with high adhesion capabilities.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.





