Introduction
According to the World Health Organisation antimicrobial resistance (AMR) is one of the most concerning threats to modern medicine [1]. Surveillance has been formulated by the Centers for Disease Control and Prevention (CDC) as one of the four core actions among public health strategies against AMR [2]. Both national and international AMR surveillance programs are mainly based on phenotypic resistance data of bacterial isolates from clinical routine, without detailed information on patient characteristics or patient setting [3, 4]. However, AMR prevalence varies considerably between different patient populations, a fact which is not adequately represented in current surveillance systems. Examples for high-risk populations include patients transferred from abroad or residents of long-term care facilities (LTCF) [Reference Strausbaugh5, Reference Mutters6]. These high-risk groups could potentially act as reservoir for the distribution and spread of AMR within healthcare networks [Reference van den Dool7].
Knowing the prevalence of AMR among these populations might not only aid clinicians in their choice of empirical antibiotic treatment, but also support infection control specialists in prioritising prevention measures or guide public health authorities in planning future screening studies. In this systematic review, we aimed to summarise patient screening data for the most important antibiotic resistant pathogens within different patient settings in Switzerland, to identify screening gaps and to interpret these findings in the context of resistance data from neighbouring countries.
Methods
Aims
The primary study aims were (i) to summarise prevalence data on antibiotic resistance from studies performed in different patient settings in Switzerland between the year 2000 and 2017 and (ii) to identify gaps in surveillance for important patient populations. We focussed on studies reporting on carbapenemase-producing Enterobacteriaceae (CPE), extended-spectrum β-lactamase (ESBL)-producing or extended-spectrum cephalosporin resistant (ESC-R) Enterobacteriaceae, pathogens harbouring the mobilised colistin resistance (MCR)-gene, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE). These five resistant pathogen groups (subsequently simply called pathogens) – accounting for almost two thirds of deaths caused by AMR in the US – were chosen based on their presumed importance for Switzerland [2].
The following settings are defined: acute/intensive care (i.e. universal and targeted admission screenings, other screenings), outpatients, LTCF and specific risk groups (i.e. refugees, travellers, people in contact with livestock, intravenous drug users [IVDU] and others). Studies in the paediatric population were analysed separately. This study is being reported according to the PRISMA guidelines [Reference Moher8].
Design and study criteria
We performed a systematic literature search for studies meeting all of the following inclusion criteria: (i) studies (i.e. point-prevalence, cross-sectional and interventional studies, admission screenings, cohort and case-control studies) reporting prevalence data among specific patient populations; (ii) studies performed in Switzerland and (iii) majority of data collected after 1999. Studies on patients of all ages colonised with any of the five pathogen groups of interest at any body site (i.e. any screening method) were included.
The following exclusion criteria were applied: studies not including prevalence data (e.g. comments, reviews or editorials, case reports or outbreak reports without additional screenings); studies reporting AMR prevalence among clinical isolates but not among patients; studies focussing on animal or environmental samples; studies reporting only data on molecular epidemiology and studies including data from other countries, where the Swiss data were not presented separately.
The following microbiologic definitions had to be met:
• CPE: any Enterobacteriaceae with detection of a carbapenemase gene
• ESBL/ESC-R: any Enterobacteriaceae with non-susceptibility to a 3rd/4th-generation cephalosporine OR detection of an ESBL gene by polymerase chain reaction
• MCR: any pathogen with detection of a mcr-gene
• MRSA: S. aureus with non-susceptibility to oxacillin OR detection of mecA gene
• VRE: any Enterococcus faecalis/faecium with non-susceptibility to vancomycin OR documentation of vanA, vanB or vanC positivity
Search methods
A professional librarian performed a literature search in the Pubmed, MEDLINE and Embase databases (from 1 January 2000 to 5 May 2017). We used both medical subject headings and text word terms for AMR or each of the five key pathogens AND Switzerland (Supplements Table S1). Furthermore, conference proceedings of the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), 2015 to 2017, and the Joint Annual Meeting of the Swiss Society for Infectious Diseases (SSI), 2013–2017, were screened. Bibliographies of included records were screened for relevant articles. No language restrictions were applied. Studies which met inclusion criteria underwent full-text review. Abstract screening and full-text review were performed by two independent reviewers. Reviewers were not blinded for author names, institution names or the journal name.
Data extraction and management
The following data are abstracted from the selected studies: study design, study year(s), geographic location within Switzerland (French vs. German vs. Italian speaking regions), patient setting (see above), additional particular patient characteristics (if any), type of antibiotic resistance including resistance mechanism (if available) and screening sites. Every study was assigned a time period (i.e. 2000–2005; 2006–2011; 2012–2016), depending on when the majority of the study data were generated. All data were double entered by two independent reviewers and any discrepancies were resolved by consensus. Because of presumed heterogeneity between studies, no pooled prevalence estimate was calculated. Prevalence data were plotted by type of pathogen and study setting (this analysis was only performed for CPE, ESBL and MRSA because of the small number of studies for MCR-producers and VRE).
Results
We identified a total of 791 unique references and 1554 conference abstracts. One-hundred and eighteen records underwent full-text review and 46 records, thereof eight conference abstracts, were included in our review (Fig. 1). A descriptive summary of the available literature by patient setting is given below. Details of included studies, separated by Gram-negative and Gram-positive pathogens, are presented in Tables 1 and 2. For identification of surveillance gaps, studies are plotted in Figure 2, stratified by pathogen, patient setting and time period.
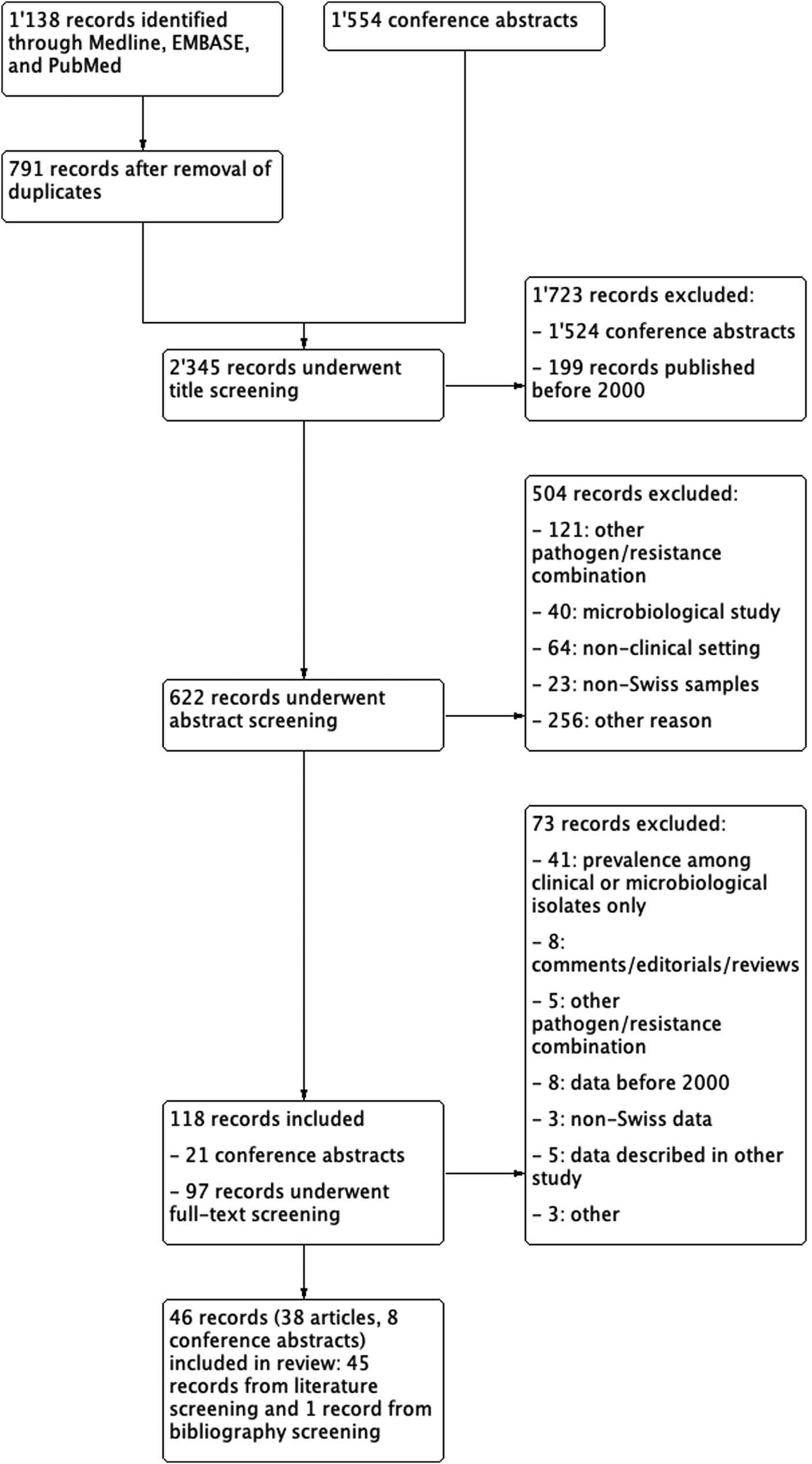
Fig. 1. Results of literature search and flow-diagram.
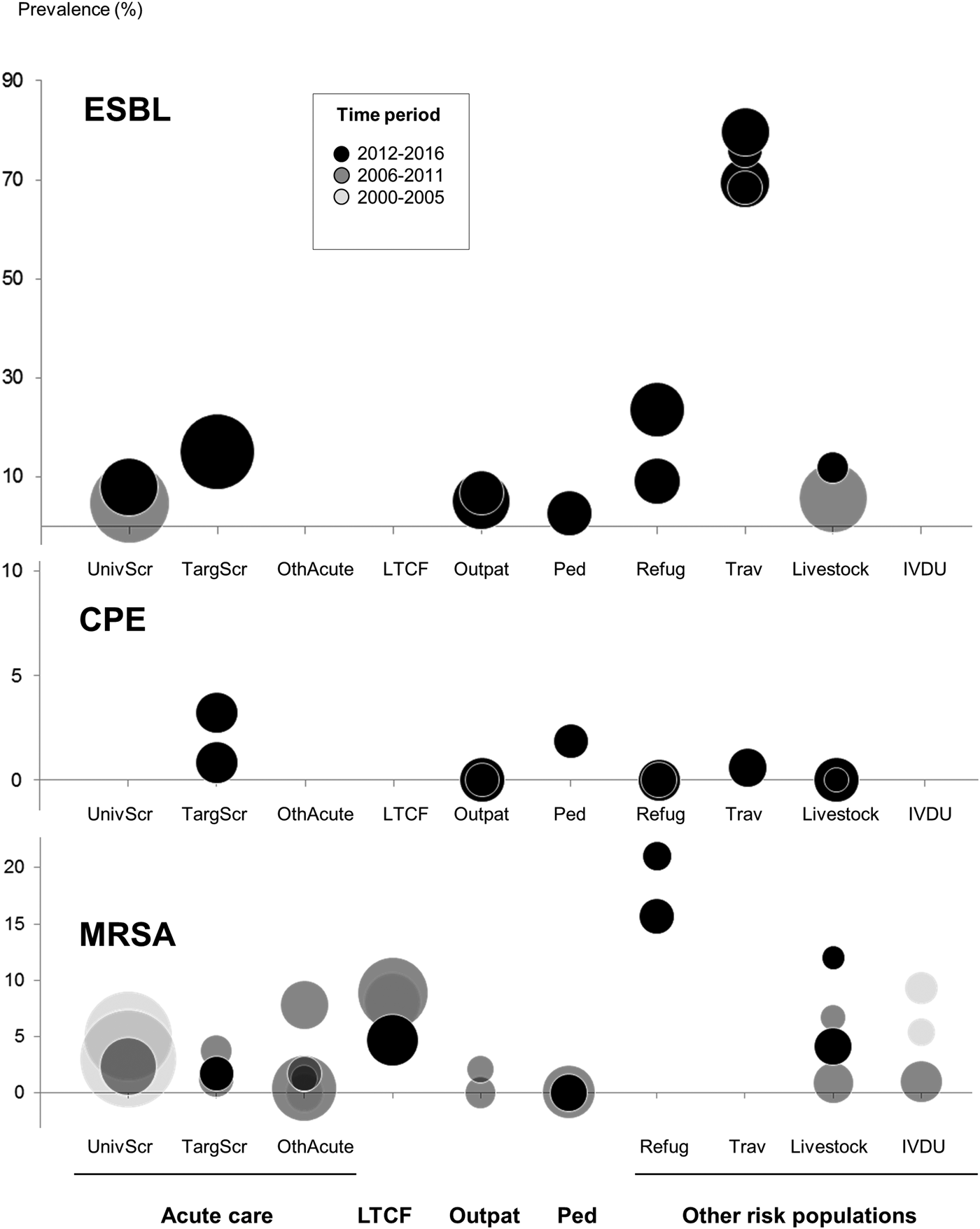
Fig. 2. Prevalence data per patient setting and time period in Switzerland for ESBL, CPE and MRSA. Every circle represents a single study; circle diameter correlates with the study sample size. Please note the different scaling of the y-axis for the CPE, ESBL and MRSA. Acute/intensive care (UnivScr, Universal admission screening; TargScr, Targeted admission screening; OthAcute, Other inpatients); LTCF, Long-term care facility; Outpat, Outpatients; Ped, Paediatric patients; other risk populations (Refug, Refugees; Trav, Returning travellers; Livestock, people working in livestock industry; IVDU, Intravenous Drug Users).
Table 1. Prevalence of antibiotic resistant Gram-negative pathogens among different patient populations in Switzerland
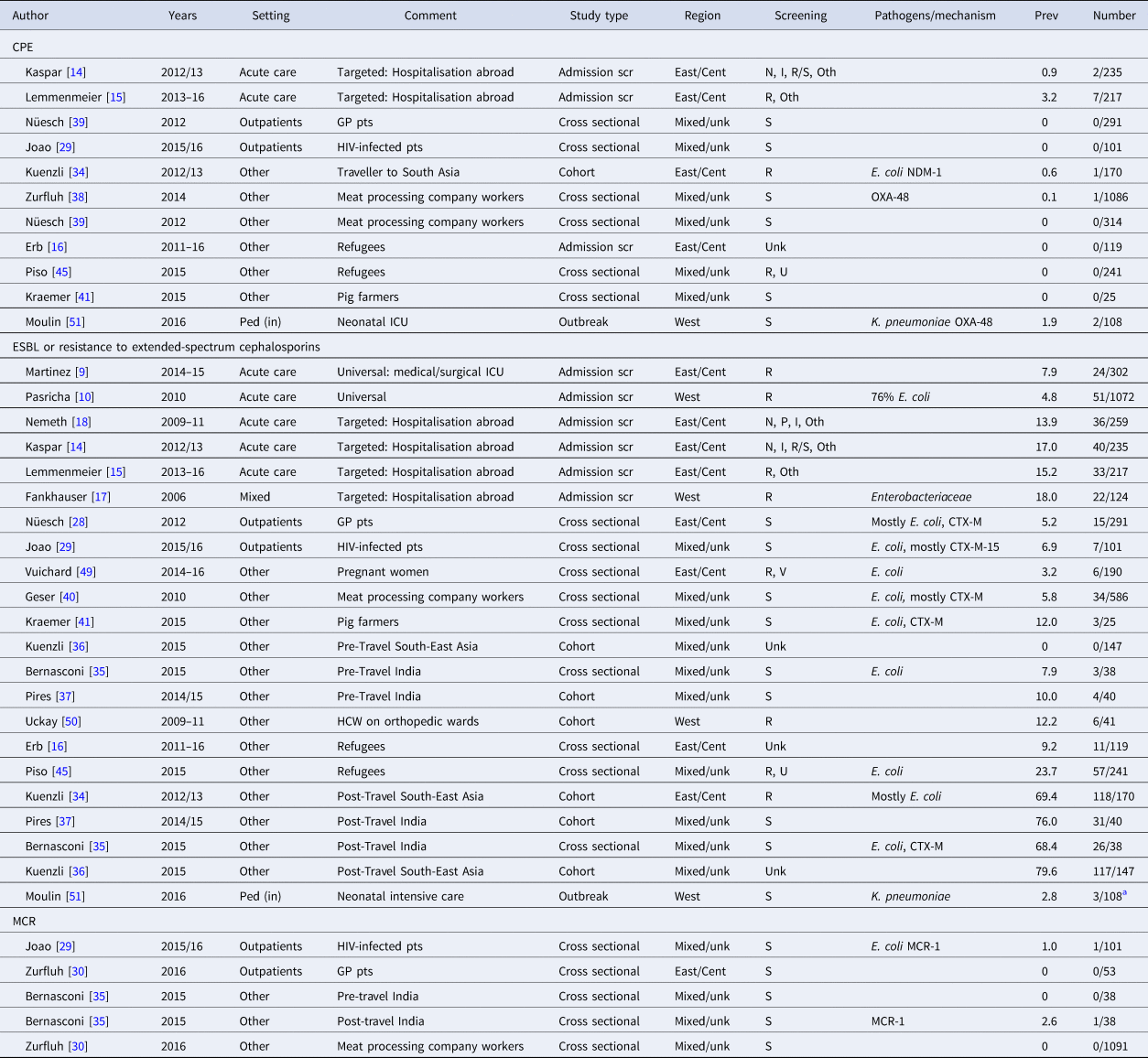
Prev, prevalence (in %); Pt, patient; N, nasal; P, pharyngeal or throat; I, inguinal or groin; S, stool or faecal; R, rectal or perineal; U, urine; V, vaginal, Oth, others sites (depending on clinical picture); Unk, unknown; Ped (in), pediatric inpatients; Scr, screening; Cent, central; GP, general practitioner; HCW, healthcare worker.
a Result from contact screening, in addition three neonates were involved in the actual outbreak.
Table 2. Prevalence of antibiotic resistant Gram-positive pathogens among different patient populations in Switzerland
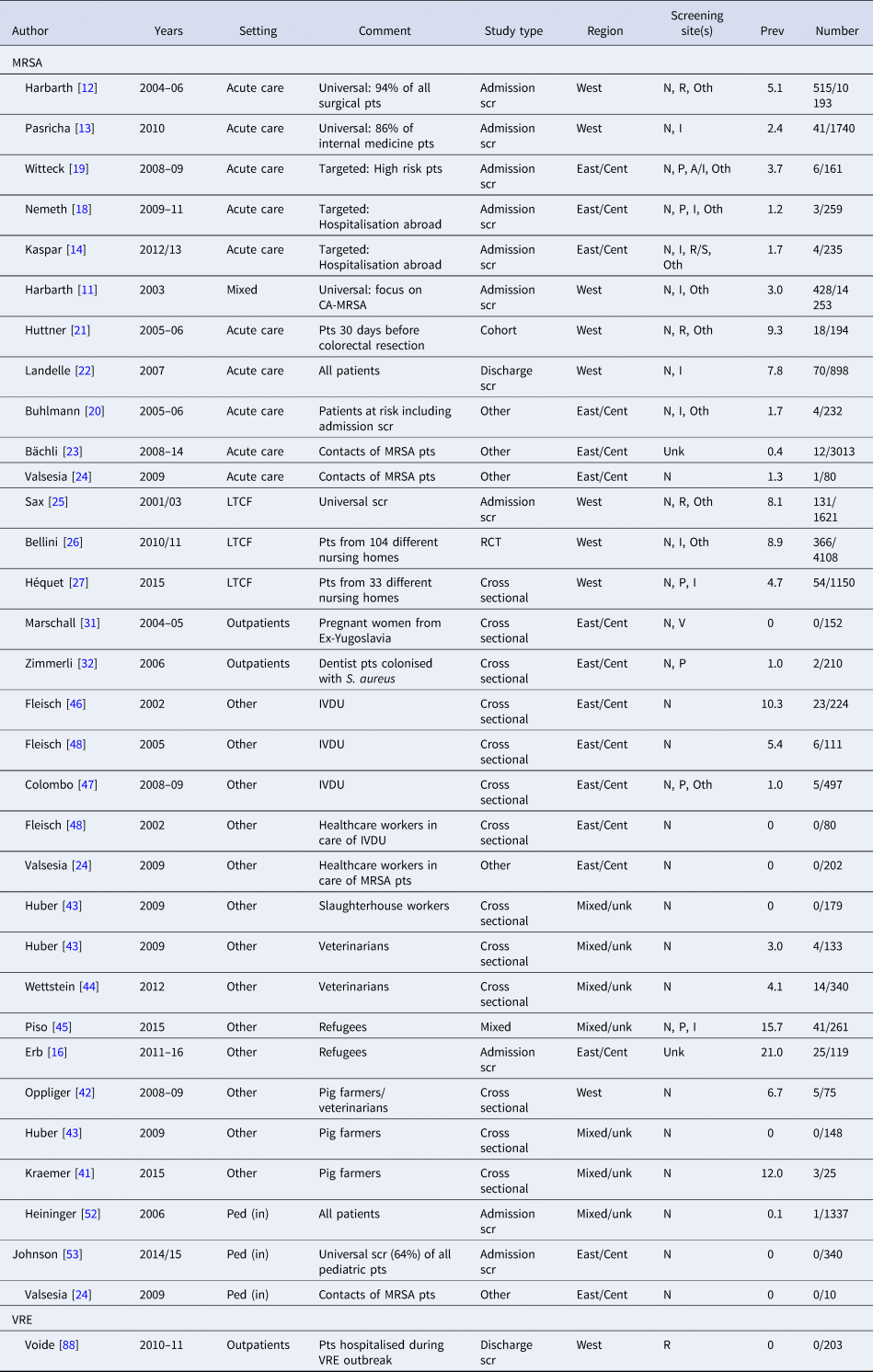
Prev, prevalence (in %); Pt, patient; N, nasal; P, pharyngeal or throat; A, axillary; I, inguinal or groin; S, stool or faecal; R, rectal or perineal; U, urine; V, vaginal, Oth, others sites (depending on clinical picture); Unk, unknown; Ped (in), pediatric inpatients; Scr, screening; Cent, central; LTCF, long-term care facility; CA, community-acquired; IVDU, intravenous drug users; RCT, randomised controlled trial.
Acute care
Universal screenings
Data from universal hospital admission screenings (i.e. screening of all patients upon hospital admission) are available for ESBL and MRSA. For ESBL, prevalences of 5% (2010) and 8% (2014/2015) have been reported [Reference Martinez9, Reference Pasricha10]. For MRSA, several older studies from the Geneva University Hospital showed prevalences of 3% (2003, mixed population) [Reference Harbarth11], 5% (2004–2006, surgical patients) [Reference Harbarth12] and 2% (2010, internal medicine) [Reference Pasricha13]. In the study from 2003, 13 of 428 MRSA isolates were classified as community-acquired (CA)-MRSA, thereof five with production of Panton-Valentine leukocidin (PVL) [Reference Harbarth11]. No data are available for other pathogens.
Targeted admission screenings
Targeted screenings (i.e. screening of high-risk patients upon admission, mostly those transferred from abroad) have shown low CPE prevalences between 1% (2012/2013) and 3% (2013–2016) [Reference Kaspar14, Reference Lemmenmeier15]. For ESBL, these numbers have ranged between 14% and 21% [Reference Kaspar14–Reference Nemeth18]. For MRSA, targeted screenings performed in the German-speaking part revealed low prevalence rates between 1% and 3% [Reference Kaspar14, Reference Nemeth18–Reference Buhlmann20].
Other screening studies
One study from 2005/2006 screened patients before colorectal surgery, whereof 9% were MRSA positive [Reference Huttner21]. Another study found a MRSA prevalence of 7% among all discharged patients from a tertiary care hospital [Reference Landelle22]. Both studies were performed in the Western part of Switzerland. Two more recent studies from the German part, evaluating MRSA prevalence among hospitalised contacts of known MRSA carriers, showed prevalence rates of only 0.4% (2008–2014) and 1.3% (2009) [Reference Bächli23, Reference Valsesia24].
Long-term care facilities/geriatric hospitals
For CPE or ESBL-producers, no screening data are available from LTCFs. For MRSA, prevalence in geriatric patients upon hospital admission was reported to be 8% in 2001/2003 [Reference Sax25]. Screening of nursing home residents in 2010/11 showed a similar prevalence of 9%, with a decreasing trend over time (5% in a follow-up study from 2015) [Reference Bellini26, Reference Hequet27]. All these studies were performed in the French speaking part of Switzerland.
Outpatients
No CPE carriers were detected in two cross-sectional studies among primary care (2012) and HIV-infected patients (2016) [Reference Nuesch-Inderbinen28, Reference Joao29]. The same studies also assessed ESBL carriage, showing a prevalence of 5% and 7% [Reference Nuesch-Inderbinen28, Reference Joao29]. Two cross-sectional studies looked at MCR prevalence among outpatients. Whereas the one study among HIV patients found 1% to be colonised with an MCR-1 producing E. coli [Reference Joao29], the other study did not identify any cases [Reference Zurfluh30]. Other screening studies among outpatients have shown MRSA prevalence rates of 0% (pregnant women from former Yugoslavia), 1% (dental care patients) and 2% (patients with skin infections) [Reference Marschall, Durig and Muhlemann31–Reference Kronenberg33].
Other specific risk groups
Returning travellers
Screening for CPE identified only 1 carrier of NDM-1 E. coli (0.6%) among returning travellers from South Asia (2012/2013) [Reference Kuenzli34]. ESBL-carriage was much more common and ranged between 68 and 80% (2012–2015) [Reference Kuenzli34–Reference Pires37]. For MCR, 1 out of 38 (3%) travellers to India was found to have acquired an MCR-1 harbouring E. coli during travel [Reference Bernasconi35].
People in contact with livestock
A dedicated group of Swiss investigators has studied the presence of resistant pathogens among workers of a meat processing factory. In one study, one out of more than 1000 (0.1%) workers was colonised with an OXA-48-producing E. coli (2014), whereas no CPE were detected in a similar study 2 years earlier [Reference Zurfluh38, Reference Nuesch-Inderbinen39]. For ESBL, prevalence was 6% in the same population (2010) [Reference Geser40]; a study from 2016 regarding colonisation with MCR-producers revealed no positive sample [Reference Zurfluh30]. Among pig farmers, no CPE were detected in 2015 whereas ESBL-prevalence was relatively high at 12% [Reference Kraemer41]. MRSA prevalence among pig farmers was 6.6% in 2008 [Reference Oppliger42], 0% in 2009 [Reference Huber43] and 12% in 2015 [Reference Kraemer41]. No MRSA was detected among slaughterhouse workers (2009), whereas 3% and 4% of screened veterinarians were MRSA-positive (2009 and 2012) [Reference Oppliger42–Reference Wettstein Rosenkranz44].
Refugees
Two studies have assessed carriage of resistant pathogens among refugees. One study which screened asylum seekers presenting at a tertiary care hospital (2014/2015) showed an MRSA-prevalence of 21%, whereas ESBL- and CPE-prevalence was 9% and 0%, respectively [Reference Erb16]. Another cross-sectional study (2015) performed in four refugee centres found a MRSA-prevalence of 16%, ESBL-prevalence of 24% and again no CPE carriers [Reference Piso45].
Intravenous drug users
Several studies have looked at MRSA prevalence among IVDU. Whereas prevalence was as high as 10% around the year 2000 [Reference Fleisch46], the number dropped to 5.4% in 2005 and to 1% in 2008/2009 [Reference Colombo47, Reference Fleisch48].
Other adult populations
For ESBL, prevalence varies greatly by risk population: 3% (6/190) in pregnant women [Reference Vuichard Gysin49]; 0–10% in individuals before travel [Reference Bernasconi35–Reference Pires37] and 12% among health-care workers [Reference Uckay50]. The MRSA prevalence rate among healthcare workers in charge of MRSA patients was 0% (2002 and 2009) [Reference Valsesia24, Reference Fleisch48].
Pediatric population
During an outbreak of ESBL-producing Klebsiella pneumoniae in a neonatal intensive care unit 108 patients were screened, whereof six were positive. Unexpectedly, 2% were also positive for OXA-48 producing K. pneumoniae [Reference Moulin51]. For MRSA, admission screening of high-risk patients found prevalence rates of 0.1% (2006) and 0% (2014/2015) [Reference Heininger52, Reference Johnson, Frei and Heininger53]. Another study from 2009 screening hospitalised contacts of pediatric MRSA patients confirmed this very low prevalence [Reference Valsesia24].
Discussion
We comprehensively summarised the available literature on AMR screening data with regard to different patient settings in Switzerland. Data in this review confirm the high prevalence of ESBL among patients transferred from abroad and returning travellers; refugees are at high risk for both ESBL- and MRSA-carriage. In general, little information is available from LTCFs and the pediatric population; prevalence data on emerging pathogens such as CPE and VRE are scarce.
AMR is primarily a concern in acute and intensive care patients due to severe underlying illnesses, antibiotic pre-treatment and medical interventions predisposing for hospital-acquired infections [Reference Cronin54–Reference Tumbarello57]. According to a nation-wide survey in Swiss healthcare facilities, 83% of institutions are currently performing targeted admission screenings for multidrug-resistant pathogens. Considerable heterogeneity exists regarding target populations and screening methods [Reference Martischang58]. Data from targeted admission screenings in Switzerland are comparable to numbers in Germany, where similar carriage rates among patients transferred from abroad for resistant Gram-negatives (13% vs. 14–17% in our review) and for MRSA (4% vs. 1–4% in our review) were found [Reference Mutters6]. ESBL-prevalence from universal admission screening was 8% in a study included in our review, which is comparable to the 10% prevalence found in a German study [Reference Martinez9, Reference Boldt59]. One of the most urgent antibiotic resistant threats, coming along with increased morbidity and mortality in infected patients, are CPE [Reference Dautzenberg60]. Looking at neighbouring countries, CPE prevalence in acute care settings varies considerably. Prevalence was reported to be as low as 0.1% upon hospital admission in Germany [Reference Hamprecht61] and 0.4% among hospitalised acute care patients in southern France in 2012 [Reference Pantel62]. In Italy, 8% of clinical Enterobacteriaceae isolates from inpatients were carbapenem-resistant in 2013 [Reference Giani63]. In Switzerland, according to national laboratory-based surveillance data, absolute numbers of CPE have been increasing in recent years, with OXA-48 being the most commonly reported carbapenemase followed by K. pneumoniae carbapenemase [Reference Ramette64]. The fact that only 64% of Swiss healthcare institutions are actually performing targeted CPE admission screenings is worrisome [Reference Martischang58]. Except from admission screenings, no prevalence data exist from Swiss acute care hospitals for these primarily nosocomial pathogens. However, modelling studies for the English hospital networks have shown that, because of the high number of patient transfers within the own healthcare network, the absolute risk of CPE introduction is higher for patients transferred within than those transferred from outside these networks, even if the local CPE prevalence is low [Reference Donker65]. In accordance with the conclusions drawn by the authors of this study, we think that further studies are needed to evaluate if CPE emergence in Switzerland is merely due to an increase of imported cases or if patients are increasingly acquiring CPE in Swiss hospitals. Regarding VRE, no screening studies have been published until date of this literature search. Of note, VRE have lately become a pressing issue in Switzerland, with multiple outbreaks having been reported within and between healthcare facilities [Reference Wassilew66, Reference Abdelbary67]. It remains to be seen if VRE will become endemic in this patient setting as it has for certain regions in Germany [Reference Remschmidt68].
An important finding of our review is the scarcity of resistance data from Swiss LTCFs. This is concerning, because (i) LTCFs have been recognised as highly endemic settings for resistant pathogens already more than two decades ago [Reference Strausbaugh5, Reference Wiener69] and (ii) a recent laboratory-based study from Swiss LTCFs showed a clear increase in E. coli isolates resistant to extended-spectrum cephalosporins over the last decade [Reference Kohler70]. Looking at neighbouring countries, data from LTCFs in Italy show that the prevalence of ESBL-producers and CPE is as high as 64% and 6%, respectively [Reference Giufre71, Reference Aschbacher72]. A point prevalence study from 2015 performed in an Austrian LTCF reported a prevalence of 13% for ESBL, whereas no CPE were found [Reference Zollner-Schwetz73]. In Germany, data from 2012 among nursing home residents showed a prevalence of 27% for ESBL, 9% for MRSA and 3% for VRE [Reference Heudorf74]. Only a minority of hospitals in Switzerland are routinely screening patients residing in LTCFs upon hospital admission [Reference Martischang58]. Whether this strategy misses a relevant number of patients carrying resistant pathogens is currently unknown.
Similar to our data, a recently published review on AMR among European migrants found a high prevalence of MRSA (8%) and resistant Gram-negative bacteria (27%), concluding that living conditions, access to healthcare and AMR detection should be improved in this high-risk population [Reference Nellums75]. In line with our findings, data from Germany suggest that ESBL-colonisation seems to be common among refugees, whereas CPE are rarely detected [Reference Ehlkes76]. Data from other countries as well as a recent review confirm the high prevalence of particularly resistant Gram-negative pathogens among returning travellers [Reference Lubbert77–Reference Ruppe, Andremont and Armand-Lefevre80]. None of the studies in our review performed MRSA screening among returning travellers. However, a multicentre study from different European cities – including a Swiss study centre – reported an MRSA prevalence of 14% (2019) among returning travellers with skin and soft tissue infections, with Latin America as travel destination being a risk factor for MRSA carriage. A large proportion of these isolates were PVL-producing CA-MRSA [Reference Nurjadi81]. Interestingly, although MRSA rates are generally decreasing in Switzerland [Reference Olearo82], the prevalence among pig farmers – who are likely to be colonised with livestock-associated MRSA – has increased between 2009 and 2015. This finding is in line with reports from Germany, showing increasing trends for LA-MRSA while overall MRSA rates are declining [Reference Schaumburg83, Reference Walter84]. Until now, CPE and MCR are not commonly detected in these settings in Switzerland. Again, no data exist for VRE.
Studies on AMR screening from the pediatric population are rare in Switzerland. National laboratory-based surveillance data suggest that the prevalence of ESBL and MRSA among clinical isolates is similar to that of the adult population [Reference Olearo82, Reference Kronenberg85, Reference Buetti86]. Of note, the unexpected finding of two neonates carrying OXA-48-producing K. pneumoniae in Switzerland is worrying and warrants further study [Reference Moulin51]. Similar to adult refugees, pediatric refugees are at particular risk of carrying resistant pathogens, as shown recently in two German studies [Reference Ehlkes76, Reference Tenenbaum87].
Our study has some limitations. First, the heterogeneity among included studies (e.g. definitions of risk for targeted admission screenings, different screening sites) precluded us from calculating a pooled prevalence or from statistically assessing trends over time. In this context it is particularly important to note that clinical microbiology breakpoints have changed during the observed time period. Second, although we performed a thorough literature search we might have missed studies, especially those only presented as abstracts in conferences which were not on our screening list. Third, some of the most recent publications were only available in an abstract form. Nonetheless, we included these studies as we would have otherwise missed the most recent data in this rapidly changing field.
In conclusion, this review confirms data from other countries showing high prevalence of ESBL-carriage among patients transferred from abroad and returning travellers; refugees are at risk for both ESBL and MRSA. There is a scarcity of AMR prevalence data from Swiss LTCFs and the pediatric population. Also, screening data are generally scarce for emerging pathogens such as CPE or VRE, which often spread within and between acute care facilities. We encourage epidemiologists and public health authorities to consider these gaps in the planning of future surveillance studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268819001523.
Acknowledgements
The authors would like to thank Martina Gosteli, PhD, Main Library University of Zurich, for performing the literature search.
Financial support
The study has been funded by the Swiss Federal Office of Public Health (disposition number 17.008726).
Conflict of interest
None.








