INTRODUCTION
Salmonellosis, a leading cause of foodborne illness, most often presents as acute gastroenteritis, but may result in septicaemia and death in vulnerable hosts such as infants and the elderly. It is a nationally notifiable disease that causes an estimated 15 300 hospitalizations and 600 deaths annually in the United States alone [Reference Voetsch1, Reference Olsen2]. The >2500 serotypes of Salmonella enterica described have diverse reservoirs and epidemiology. Salmonella surveillance in the United States is a passive, laboratory-based system [Reference Olsen2] conducted by the Centers for Disease Control and Prevention (CDC). We noted marked age and sex differences in the epidemiology of Salmonella infections by serotype [3], and, additionally, that Salmonella bacteriuria in elderly women has increased [Reference Sivapalasingam4]. To investigate whether these observations correspond to broader trends, we explored the epidemiology of non-typhoidal salmonellosis during the period 1968–2000.
METHODS
First, we examined data on Salmonella isolates reported to the National Salmonella Surveillance System (NSSS). Begun in 1962, this system collects weekly reports of Salmonella isolates serotyped in public health laboratories and catalogues the age, sex, county of residence, and clinical source of the isolates. Since 1967, the data have been gathered in a digital database [Reference Olsen2] and have been transmitted via the Public Health Laboratory Information System since 1990 [Reference Bean5]. We restricted our analysis to the 30 most common non-typhoidal Salmonella serotypes. We also excluded isolates from urine, because women are anatomically more susceptible to urinary tract infections and because we have previously identified a sex-specific trend [Reference Sivapalasingam4]. We excluded isolates for which either age or sex information was not available, since these were our primary variables of interest.
We examined and plotted the median age at diagnosis of salmonellosis by sex over time for the 30 most common Salmonella serotypes. We then linked the NSSS database to corresponding census data from the years 1968–2000 to calculate incidence rates for all 30 serotypes combined within age, sex, and period strata. Using Poisson regression, with standard errors adjusted for a deviance-based measure of overdispersion, we modelled the incidence rate as a function of age (<5, 5–19, 20–39, 40–59, ⩾60 years), sex, and period (1968–1980, 1981–1990, 1991–2000). We used Poisson regression to calculate an overall year and age-adjusted female-to-male incidence rate ratio (FMRR) as well as adjusted FMRRs for infection with each serotype. Finally, we examined the relationship between these 30 serotype-specific FMRRs and the median age of infection. This analysis included a weighted correlation between the log FMRR and median age, weighting each serotype inversely proportional to the variance of the log FMRR. All statistical analyses were performed using SAS software (version 9.1, SAS Institute, Cary, NC, USA) and reported P values are two-sided.
To assess the possibility of differential reporting contributing to the observed findings, we used the 1996–1997, 1998–1999, and 2000–2001 population-based Foodborne Diseases Active Surveillance Network (FoodNet) to compare reported rates of diarrhoea, medical visits among those reporting diarrhoea, and stool cultures among those reporting medical visits for females vs. males of different ages [Reference Jones6]. Begun in 1996, FoodNet actively collects data regarding foodborne illness from 10 sites which together included 15·2% of the US population in 2000 [Reference Olsen2].
RESULTS
Sex- and age-specific differences in salmonellosis
There were 1 154 323 isolates of Salmonella reported to NSSS from 1968 to 2000. Of these, 1 051 691 were serotyped, and 1 028 239 were found to be serotypes other than S. Typhi and S. Paratyphi A, B or C. Of these, 934 432 (91%) belonged to one of the 30 most frequently reported non-typhoidal Salmonella serotypes; 917 246 (98%) were from sources other than urine. Information about age and sex was available for 725 339 of these reported isolates.
Over the 33-year period 1968–2000, the total number of reports of salmonellosis in females and males was nearly equal (363 844 vs. 361 495, respectively). However, these figures hide important sex- and age-specific differences. For example, the median age at diagnosis generally increased over time and was consistently higher in females than in males over these 33 years (Fig. 1). Furthermore, the gap between the median age of females vs. males widened. In 1968, the median age of infected males was 4 and of females 7 years (gap of 3 years) whereas in 2000 the corresponding ages were 17 and 25 years respectively (gap of 8 years). These observations prompted us to link the NSSS data with age and sex distributions during those years in the United States, and to explore the patterns further by calculating population-adjusted incidence rates.

Fig. 1. Median age at diagnosis of salmonellosis for females (——) and males (– – –) over the 33-year period (1968–2000).
To illustrate patterns in incidence rates by age and period, we combined sex-specific data and calculated age-specific rates. Most striking was the high incidence (44·3/100 000) in the youngest age group (<5 years) relative to the rates in older age groups (Fig. 2). Incidence rates remained stable in children aged <5 years over the three decades, but increased in adults by 50–100% from the period 1968–1980 to 1981–2000.

Fig. 2. Incidence rates of salmonellosis by age group: ········, 1968–1980; – – –, 1981–1990; ——, 1991–2000.
When all age groups were combined, the incidence rate ratio of salmonellosis was almost identical in females and males (FMRR 0·99). However, the FMRR varied by age with FMRRs <1 in children (⩽19 years) and >1 in adults (Fig. 3). More specifically, we found the FMRR to be 0·92 for the <5 years age group, 0·85 for 5–19 years, 1·09 for 20–39 years, 1·23 for 40–59 years, and 1·08 for ⩾60 years. These results were confirmed in our Poisson regression modelling. In the full model with all main effects, two-way, and three-way interactions, both the three-way age×sex×period interaction and the two-way sex×period interaction were not significant (P=0·29 and P=0·89, respectively). Omitting these from the model left only age×sex and age×period interactions significant (both P<0·0001). The final model suggests that the FMRR varied significantly by age but that this relationship did not change over the three time periods studied. The five age-specific FMRRs from this final model are almost identical to the crude FMRRs reported above (Table 1). The same result was reached when we repeated the modelling by categorizing age into nine categories (Figs 2 and 3).

Fig. 3. Female-to-male incidence rate ratio (FMRR) by age group: ········, 1968–1980; – – –, 1981–1990; ——, 1991–2000.
Table 1. Adjusted female-to-male incidence rate ratio by age group, with 95% confidence intervals, for infection with one of the 30 most common non-typhoidal Salmonella serotypes, 1968–2000
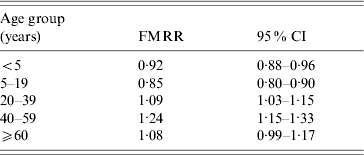
FMRR, Female-to-male incidence rate ratio; CI, confidence interval.
Serotype-specific observations
The overall FMRR for salmonellosis of 0·99 hides not only important age differences but also serotype-specific ones (Table 2). For example, during 1968–2000, the serotype-specific FMRR ranged from 0·87 (S. Mississippi) to 1·25 (S. Senftenberg). Nineteen of the 30 serotypes were more commonly isolated from females than males; however, S. Typhimurium, the most common serotype, was isolated more frequently from males (FMRR 0·94). The median age of those infected also varied by serotype. The serotype-specific median age ranged from 2 years for S. Derby to 29 years for S. Senftenberg. Additionally, we found a positive correlation between the serotype-specific FMRRs and median ages of infection (correlation 0·76, P<0·0001) (Fig. 4). In the age groups <5 years, 5–19 years, 20–39 years, 40–59 years, and ⩾60 years, the number of serotypes with FMRRs >1 (female predominance) were 0, 3, 29, 30, and 27, respectively.

Fig. 4. Female-to-male incidence rate ratio (FMRR) by median age at diagnosis for Salmonella serotypes (areas of circles are proportional to number of cases) isolated in the United States during 1968–2000.
Table 2. Median age and adjusted female-to-male incidence rate ratio for infection with one of the 30 most common non-typhoidal Salmonella serotypes, 1968–2000Footnote *

FMRR, Female-to-male incidence rate ratio.
* Non-urinary isolates of non-typhoidal Salmonella.
Reported diarrhoea, health-seeking, and culturing practices
Overall, 5·1% (1039/20 404) of females and 4·2% (620/14 897) of males [risk ratio (RR) 1·22, 95% confidence interval (CI) 1·11–1·35] reported diarrhoea at the time of the FoodNet surveys (P<0·0001). Reported diarrhoea was higher in females in all but the <5 years age group; in this group 7·4% (59/801) of females and 10·9% (64/588) of males (RR 0·68, 95% CI 0·48–0·95) reported diarrhoea (P=0·02). In ⩾5-year-olds, the reported rate in females was 5·0% (980/19 603) whereas that in males was 3·9% (556/14 309) (RR 1·29, 95% CI 1·16–1·42); this difference was highly significant (P<0·0001).
Only a subset of ill individuals reported seeking medical care for diarrhoea, and only a fraction of them reported submitting a stool culture. Females and males reported seeking care for diarrhoea at identical rates [19·2% (200/1039) for females; 19·2% (119/620) for males; RR 1·00, P=0·97). In no age group was the RR for care-seeking significantly different from 1. Of the few individuals who reported submitting a stool culture, females were not statistically more likely (10%, 32/200) than males (7·2%, 23/119) to submit one (RR 1·39, P=0·45).
DISCUSSION
National Salmonella surveillance data from the last third of the twentieth century show an unexplained age-related excess in Salmonella-related morbidity in older females, especially during the middle adult years. Boys, however, appeared to be at a higher risk for salmonellosis than girls. Within individual serotypes, the median age at diagnosis of salmonellosis and the female-to-male incidence rate ratio are positively correlated. Serotype-specific differences in the FMRR of Salmonella infection appeared to be related to the age of infection: some serotypes affected young males disproportionately, others infected older females. Finally, although the sex difference in median age at diagnosis was present 30 years ago, it continued to widen over time as the overall median age of infection and of the population increased.
The excess reported incidence of salmonellosis in adult women has several possible explanations. Review of survey data from FoodNet does not suggest culturing of stools or reporting of results disproportionate to that of diarrhoea [Reference Jones6]. Indeed, consistent with our findings for salmonellosis, those data suggest that the incidence of diarrhoeal illness in general is higher in women than men but lower in young girls than boys, a finding which has repeatedly appeared in surveys conducted in several developed countries independent of different response rates (35–84%) [Reference Scallan7]. If there is a real difference in incidence between women and men, is this because women are more likely to be exposed to the organism or are they more likely to become ill if exposed, regardless of the source?
That nearly all serotypes of Salmonella were more common in adult women suggests that there may be an underlying biological difference in host susceptibility. Pregnant women are particularly vulnerable to a variety of infections such as malaria, hepatitis (especially B and E), cytomegalovirus, and paralytic poliomyelitis [Reference Mims, Nash and Stephen8], and disease is often especially severe in these hosts. Citing a study in which 28 XXX women had higher mean IgM levels than XX women, and women had higher levels than men [Reference Rhodes9], some have also posited that the presence of two or more X chromosomes makes females more genetically and immunologically robust. It has also been suggested that women have oestrogen-related higher immunoglobulin levels and mount stronger immune responses following immunization than males [Reference Verthelyi10]. One might postulate that oestrogen levels (whether endogenous or exogenous) could also contribute to higher incidence rates of salmonellosis in women, but this remains to be proven. Surveys in several developed countries have found that diarrhoea during adulthood occurs most commonly in the 25–44 years age group [Reference Scallan7]. However, the fact that the gap in incidence rates by sex is more pronounced in women aged 40–59 years than in women aged 15–39 years suggests that hypotheses tied to childrearing or to reproductive age are not likely to explain fully the differences observed. Interestingly, although rates of Salmonella bacteriuria have remained stable in older men since the 1970s, the age-adjusted rate of Salmonella bacteriuria among older women has doubled [Reference Sivapalasingam4]. This suggests that a similar difference in host susceptibility and/or exposure may underlie both phenomena.
In addition to global differences in salmonellosis by age and sex, we found substantial variation in the age of infection across individual Salmonella serotypes. Different serotypes have different animal reservoirs, the carcasses of which can contaminate or cross-contaminate foods and other vehicles. Thus, serotype-specific differences in ages of infection may be explained by different exposures, some of which may also be gender-related. Non-typhoidal Salmonella species inhabit the intestinal tracts of mammals, birds, and reptiles. Outbreak investigations have demonstrated that most human infections result from ingestion of foods of animal origin that are contaminated with Salmonella species [Reference Bean5], and that infection with different serotypes can be linked to specific exposures. For example, S. Enteritidis has been associated with ingestion of raw and undercooked eggs [Reference Mishu11, Reference Marcus12], including intact eggs in the shell [Reference Mishu11], as well as with uncooked or undercooked chicken [Reference Kimura13, Reference Cowden14]. Further, eating chicken prepared outside the home and undercooked eggs inside the home have been associated with S. Enteritidis infections [Reference Marcus12]. Other common Salmonella serotypes, including Heidelberg, Montevideo, Hadar, Infantis, Typhimurium, Ohio, Thompson, Senftenberg, Berta, Saintpaul, and Enteritidis, have been isolated from poultry flocks, products, and/or their environments [Reference Roy15]. S. Typhimurium DT104 has additionally been isolated from hog and beef carcasses [Reference Larkin16]. Most of the FMRRs associated with these serotypes are >1 (female predominance), which may reflect increased exposure during preparation of meals.
In FoodNet surveys, females aged ⩾5 years reported more exposure than males to raw chicken [Reference Jones6]. Therefore, the fact that poultry-associated serotypes are seen more frequently in females aged 15–59 years is not surprising. Kendall and others found that individuals aged >65 years were more likely than younger individuals to report risky food storage and handling behaviours, including not keeping raw animal products separated from produce in the refrigerator, not using a meat thermometer, and not refrigerating foods promptly [Reference Kendall, Hillers and Medeiros17]. However, it is interesting to note that a similar female-to-male ratio has not been observed with infections due to Campylobacter, an organism that rivals Salmonella in terms of burden of illness, is also a commensal in poultry, and whose transmission is also primarily foodborne [Reference Rodrigues18]. Whether the same observations would be seen in other organisms is unclear.
In addition to food-handling practices, produce consumption may also place women at increased risk. Multiple outbreaks of Salmonella infections have been associated with fresh produce, including mangoes [Reference Sivapalasingam19], tomatoes [20], alfalfa sprouts [Reference Mahon21], cantaloupes [Reference Mohle-Boetani22], watermelon [Reference Gayler23], coriander (cilantro) [Reference Campbell24], and lettuce [Reference Weissinger, Chantarapanont and Beuchat25]. More women than men reported exposure to the implicated fruit or vegetable in many of these outbreaks. Surveys also suggest that although produce consumption has increased in both women and men in recent years, women of all ages consistently report consuming more fruits and vegetables than do men [Reference Li26].
Finally, diarrhoea in the 25–44 years age group may relate to exposure to enteric pathogens during nappy-changing and child care. Although mothers with salmonellosis can infect their infants, especially those not breast-fed [Reference Rowe27], they probably far more often contract salmonellosis from toddlers and infants with gastroenteritis [Reference Mermin28].
The relative excess of salmonellosis among young boys may also have a general, and even biological, explanation. Many have noted excess morbidity and mortality in males in early life, and have suggested that genetic susceptibility may play a role [Reference Wells29–Reference Purtilo and Sullivan32]. Examples of infections reported to be more common in male than female infants include rotavirus [Reference Ryan33], infectious hepatitis, and typhoid fever [Reference Winter31]. An Israeli review from 1966 to 1985 reported that the average male-to-female incidence rate ratio was 1·4 for viral hepatitis, 2·0 for viral meningitis, 1·1 for shigellosis, and 1·2 for salmonellosis [Reference Green34]. In the 0–4 years age group, the excess disease burden in males ranged from 16% for shigellosis to 98% for viral meningitis with narrow confidence intervals. To explore variations in male-to-female incidence ratios for different infectious diseases, Green constructed a mathematical model wherein the excess burden of disease in males could be predicted by the ratio of symptomatic to asymptomatic infection, with male predominance difficult to detect in diseases such as measles where nearly all are symptomatic [Reference Green34]. Others have noted sex differences in the reported incidence of infections in childhood and suggested that the gap may reflect a higher prevalence of immunodeficiency in males [Reference Washburn, Medearis and Childs30, Reference Schlegel and Bellanti35]. Although the prevalence of overt immunodeficiency is not high enough to explain the ratios Green observed, a milder spectrum of undiagnosed deficits could [Reference Green34]. Interestingly, Green found a reversed sex ratio in adulthood just as we did – females aged 15–44 years had a higher incidence of infectious diseases, particularly shigellosis and salmonellosis, than did males [Reference Green34]. Weinberger et al. described a relationship between age, serotype, and the likelihood of blood invasiveness of Salmonella species, but did not examine the association between sex and the other three [Reference Weinberger36].
In addition to possible increased biological susceptibility, it is likely that exposures contributed to the relative excess of salmonellosis in boys. Salmonellae are part of the normal gastrointestinal flora of reptiles and amphibians. In fact, 40% of all Salmonella serotypes have been cultured most often from reptiles, and rarely if ever from other animals or humans [Reference Mermin, Hoar and Angulo37]. Although <1% of Salmonella infections have been reported as reptile-associated historically [Reference Mermin, Hoar and Angulo37], reptile-associated serotypes are increasing in frequency whereas others are decreasing [Reference Olsen2]. Common Salmonella serotypes associated with reptile or amphibian exposure include Agona, Berta, Java, Javiana, Manhattan, Panama, Poona, Sandiego, and Typhimurium [Reference Olsen2, Reference Wells38]. The fact that most of the FMRRs associated with these serotypes were <1·0 (male predominance), and that boys have more frequently reported reptile exposure in FoodNet surveys [Reference Jones6], suggests that this epidemiological exposure may place boys at increased risk. Increased exposure to reptiles, however, would seem an improbable explanation for the observed male predominance in infants and toddlers, who are at greatest risk for salmonellosis. Rather an intrinsic or biological difference in susceptibility seems more plausible.
Limitations of our analysis include that we cannot test the explanatory hypotheses, since our analysis is observational and retrospective. Randomizing individuals of differing ages and sex to Salmonella exposure is not feasible or ethical; however, the hypotheses proposed here could be examined in larger surveys and cohort and case-control studies. The behavioural observations of the FoodNet surveys were limited to 1996 and later, and cannot be used to explain the trends over the three decades. We cannot exclude reporting bias either from the nature of the limited exposures captured or through factors associated with the respondents (e.g. those interviewed may not be representative of the general US population, those reachable by telephone may be different from those not, and women may be more willing to divulge confidential health information than males in any age group) [Reference Friedman39]. Different health-seeking behaviour may also have led to reporting bias. While this may explain reported sex differences for some infections in some age groups in some areas (e.g., young women may seek care more than men), the variety of populations from which these differences were reported suggest that true differences probably exist. Finally, the number of respondents in the FoodNet surveys who sought medical care for diarrhoea was small, which limits our ability to examine the possibility of biased culturing of stools, particularly within age strata.
In this study we have observed and robustly verified statistically an excess burden of salmonellosis in adult women relative to men during the period 1968–2000 in the United States that is related to an increase in median age at diagnosis during the same time period. We hypothesize that the propensity to contract salmonellosis includes both biologically based and behaviour-based factors throughout the lifespan. Limited data from other countries suggest that similar patterns in the epidemiology of salmonellosis exist elsewhere [Reference Cowden14, Reference Schonheyder and Ejlertsen40]. Further exploration is needed to inform preventive strategies so that continued progress may be made to reduce the burden of foodborne illness. Because only some ill persons seek care, and only about 20% submit a sample for culture [Reference Scallan7], it is estimated that each culture-confirmed case of salmonellosis represents 38 illnesses [Reference Voetsch1]. Hence, the true burden of salmonellosis with its excess burden in women may be an underestimate. Since older persons have a higher rate of bacteraemia, hospitalization, and death [Reference Weinberger36] than do younger persons, and those infected with increasingly resistant strains [Reference Molbak41, Reference Varma42] also do more poorly than those with susceptible strains, further study and targeted control efforts will be needed to curb excess Salmonella-related morbidity and mortality in women.
ACKNOWLEDGEMENTS
The Centers for Disease Control and Prevention provided financial support. Dr M. E. Reller completed this work as a Glaser Pediatric Clinical Investigator Fellow at Children's Hospital Boston.
DECLARATION OF INTEREST
None.










