Introduction
Intestinal parasitic infections are among the main causes of human morbidity and mortality, especially in developing countries (Kiani et al., Reference Kiani, Haghighi, Rostami, Azargashb, Tabaei, Solgi and Zebardast2016). According to World Health Organization (WHO) estimates, more than 2 billion people are chronically infected with at least one gastrointestinal helminth parasite. These infections have been categorized as ‘Neglected Tropical Diseases’ because they affect the poorest and most deprived communities (World Health Organization, 2005). One of these intestinal parasites is Hymenolepis nana, which is a common tapeworm that affects humans (Thompson, Reference Thompson2015). Generally, H. nana infection has a worldwide distribution, but the highest prevalence is found in the temperate regions of developing countries, where poor sanitation and bad hygiene favour its direct transmission, especially among children (Abdel Hamid et al., Reference Abdel Hamid, Eljack, Osman, Elaagip and Muneer2015).
Some drugs are available for the treatment of H. nana infection, including praziquantel, nitazoxanide and niclosamide. The drug of choice is praziquantel, due to its high cure rate, which may reach 100% (Campos et al., Reference Campos, Bressan and Evangelista1984). Conversely, the efficacies of nitazoxanide and niclosamide are variable. For instance, the cure rate with nitazoxanide was 84% even after three treatments in one clinical trial (Diaz et al., Reference Diaz, Mondragon, Ramirez and Bernal2003). Additionally, in a comparative study, the anticestodal drug niclosamide was found to be less effective against H. nana than praziquantel (Gupta & Katiyar, Reference Gupta and Katiyar1983).
Hymenolepis nana resistance against praziquantel has not been reported to date. However, there is a growing fear of developing this resistance as a logical consequence of drug abuse, dependence on a narrow range of drugs (Hoste & Torres-Acosta, Reference Hoste and Torres-Acosta2011) and poor patient compliance, especially in endemic areas where poverty and ignorance are dominant. Unfortunately, indicators of the development of praziquantel resistance by different parasites have begun to appear during recent decades. For instance, Schistosoma mansoni field isolates with praziquantel resistance have been reported in Senegal (Fallon et al., Reference Fallon, Sturrock, Niang and Doenhoff1995) and Egypt (Ismail et al., Reference Ismail, Metwally, Parghaly, Bruce, Tao and Bennett1996). Additionally, the failure of praziquantel treatment was documented among Taenia saginata-infected patients (Lateef et al., Reference Lateef, Zargar, Khan, Nazir and Shoukat2008). Therefore, there is an urgent need to search for new antiparasitic agents to overcome this problem. Although the resistance mechanisms mentioned above may operate with any new drug, the presence of several alternatives to a single drug of choice should delay the time, or decrease the chance, of emergence of resistance.
Approximately 80% of people living in endemic areas for parasitic diseases rely on medicinal plants to cover their primary healthcare needs (World Health Organization, 2000). Additionally, almost 80% of the drugs used globally either originated from natural plants or were inspired by their natural derivatives (Li & Vederas, Reference Li and Vederas2009). Therefore, the WHO traditional medicine strategy (World Health Organization, 2013) recently encouraged research studies inspired by traditional medicine to evaluate both the safety and effectiveness of widely used remedies worldwide.
One well-known medicinal herb is Artemisia absinthium. This herb is commonly known as ‘wormwood’ due to its anthelmintic properties, which have been recognized since the time of the ancient Egyptians (Padosch et al., Reference Padosch, Lachenmeier and Kröner2006). In traditional medicine, the dried whole plant and its essential oil have been used as anthelmintic, antiseptic, antispasmodic and sedative remedies (Deans & Kennedy, Reference Deans, Kennedy and Wright2002). During the past century, its use had declined due to fear of absinthism, which is a syndrome characterized by addiction, hyperexcitability, epileptic fits and hallucinations. This syndrome was attributed to thujone (a monoterpene ketone often present in the essential oil of wormwood). However, recent studies have shown that the concentrations of this substance in A. absinthium extracts are not sufficient to exceed the toxic thresholds. Additionally, thujone is less soluble in water than in ethanol. Therefore, the fears about absinthium have decreased, and in recent medical studies wormwood has started to gain attention as an alternative to conventional drugs (Lachenmeier, Reference Lachenmeier2010).
Few experimental reports have investigated the use of A. absinthium as an antiparasitic agent. For example, A. absinthium has been used against gastrointestinal ovine nematodes (Tariq et al., Reference Tariq, Chishti, Ahmad and Shawl2009), Trichinella spiralis (Caner et al., Reference Caner, Döşkaya, Değirmenci, Can, Baykan, Uner, Başdemir, Zeybek and Gürüz2008), Toxocara catti (Yıldız et al., Reference Yıldız, Başalan, Duru and Gökpınar2011), S. mansoni, Fasciola hepatica (Ferreira et al., Reference Ferreira, Peaden and Keiser2011), Trypanosoma congolense (Kifleyohannes et al., Reference Kifleyohannes, Terefe, Tolossa, Giday and Kebede2014), Trypanosoma cruzi and Trichomonas vaginalis (Martínez-Díaz et al., Reference Martínez-Díaz, Ibáñez-Escribano, Burillo, Heras-Lde, Prado, Agulló-Ortuño, Julio and González-Coloma2015). However, to the best of our knowledge, no previous studies have evaluated the use of A. absinthium against cestodes in general and H. nana in particular.
Therefore, the present study was designed to evaluate the therapeutic effects of the traditionally well-known medicinal herb, A. absinthium, against the common intestinal tapeworm H. nana. In addition to the medical importance of this parasite, H. nana is a good model that can be maintained and cultivated easily in the laboratory. In this study, in vitro and in vivo antiparasitic assays were conducted to compare the crude aqueous extract of A. absinthium to praziquantel, which is the drug of choice for the treatment of H. nana infection.
Materials and methods
Plant material and drugs
Plant material and extraction
Artemisia absinthium grows naturally on the open slopes of mountains in Saini, Egypt. The leaves of this erect plant are small, separated into three deeply lobed leaflets, and greyish-green with numerous small, pale yellow flowers. The leaves and flowers have a characteristic odour. For this study, fresh aerial parts were purchased from a local herb store in September of 2015. The plant was identified and authenticated by a botanist at the Botany Department, Faculty of Science, Menoufia University. The plant was thoroughly washed under running water and then air-dried in the shade. The dried plant was pulverized into a fine powder using an electric blender. The powdered plant material was stored in an airtight brown container at 4°C prior to extraction. In this study, the crude aqueous extract was chosen to decrease the possibility of the occurrence of systemic toxicity caused by thujone, which is more soluble in alcohol than in water. The extraction was performed according to the method of Kifleyohannes et al. (Reference Kifleyohannes, Terefe, Tolossa, Giday and Kebede2014). Briefly, 100 g of the powdered plant was soaked in 500 ml of boiled distilled water in a flask and stirred intermittently for 72 h at room temperature. The material was filtered using a sterile Whatman No.1 filter paper into a clean flask. The filtration process was repeated three times. The pooled aqueous filtrate was freeze-dried and lyophilized to obtain a yield of 8.15 g. The extract was stored in a refrigerator at 4°C until needed.
Reference drug
Praziquantel (Discocide, 600 mg; Egyptian International Pharmaceutical Industries Company (EIPICO), Ramadan City, Egypt) was used as the reference drug in the anticestodal assays. This drug was dissolved in 0.1% dimethyl sulphoxide (DMSO) (Sigma-Aldrich, St. Louis, Missouri, USA) prior to use.
Experimental animals
In the present study, Swiss albino male mice, 6–8 weeks of age and weighing an average of 20–25 g at the beginning of the experiment, were used. The mice were housed under controlled temperature and humidity conditions (25°C, 70%) and provided water and a balanced diet. An adaptation period of 2 weeks was allowed prior to commencement of the study.
Parasite maintenance
Fresh stool samples collected from patients attending Menoufia University hospitals were examined for the presence of H. nana eggs. The eggs were isolated from the stools using a saturated NaCl flotation method (Voge, Reference Voge, MacInnis and Voge1970). The collected eggs were transferred into a clean 10-ml centrifuge tube and then washed with phosphate-buffered saline solution (PBS, pH 7.2) three times, to remove any trace of salt. The sediment containing the H. nana eggs was suspended in 0.1 ml of PBS (pH 7.2) to maintain their viability. To assess the egg viability, a sample of the isolated eggs was subjected to induction of hatching by addition of sodium hypochlorite solution together with a 0.01% trypan blue solution, as described by Wang et al. (Reference Wang, Ma, Kuo and Fan1997). Hatching was observed under a microscope; the viable oncospheres were unstained. To maintain the strain in the laboratory, five mice were immunosuppressed by hydrocortisone acetate at a dose of 125 mg/kg per day orally for five consecutive days, starting on the same day as oral infection with the isolated H. nana eggs via gastric tube. The infective shell-free eggs were prepared by stirring the egg suspension with glass beads, as described by Matsuzawa et al. (Reference Matsuzawa, Nakamura, Abe and Okamoto1998). Each inoculum was adjusted to 200 eggs/0.1 ml of PBS, using a McMaster counting slide under a microscope.
Recovery of adult worms
Four weeks after infection, the mice were sacrificed and then dissected. The intestines were placed in Petri dishes with 0.9% NaCl to release adult worms from the intestinal mucus. The worms were washed several times with saline. The adult worms were examined individually under a microscope. Worms exhibiting any type of damage were isolated, and their gravid segments were homogenized in PBS to obtain the infective egg inoculum used in the in vivo study. The intact living adults were assigned to the in vitro study.
In vitro study
Living adult H. nana worms collected from freshly sacrificed mice were divided into four groups (n = 5) in separate Petri dishes with PBS, in an incubator at 37 ± 1°C. Group I served as the untreated control group. Group II was treated with the reference drug praziquantel at a concentration of 1 mg/ml. Groups III and IV were exposed to the A. absinthium crude extract at concentrations of 1 and 5 mg/ml, respectively. The extract and the reference drug were dissolved in a few drops of 0.1% DMSO, and the same amount of DMSO was administered to the control group.
The activity and survival of the adult worms were monitored under a microscope. Worm activity was determined by the presence of movement and contractions at the neck region. Worms not showing any physical movement upon gentle stimulation with a soft brush were picked up and transferred to warm PBS (40 ± 1°C). A total loss of noticeable worm movement during exposure to the warm solution was considered a sign of paralysis. Worm death was determined if stimulation by the warmer solution (~45°C) for more than 30 min did not stimulate any physical activity in the paralysed worm (Deori & Yadav, Reference Deori and Yadav2016). The experiments were repeated three times for each group.
Ultrastructure determination by transmission electron microscopic examination
Immediately after the induction of worm paralysis, the worm samples were fixed and prepared according to the method of Roy et al. (Reference Roy, Dasgupta and Tandon2008). Briefly, the control and treated worms were fixed in 2.5% glutaraldehyde buffered with 0.1 m sodium cacodylate (pH 7.2) at 4°C. Small pieces of the worm were post-fixed in 1% osmium tetroxide at 4°C for 2 h, dehydrated in an acetone series, and then embedded in Spurr's epoxy resin. Semi-thin sections were prepared and stained with 1% methylene blue in borax solution. For the transmission electron microscopy (TEM) analysis, ultrathin sections were placed on copper grids and double-stained with uranyl acetate and lead citrate. The examination was performed under the JEOL JEM 1230 transmission electron microscope (JEOL, Tokyo, Japan) operated at an accelerating voltage of 80 kV.
In vivo study
Experimental design
Forty Swiss albino mice were inoculated orally with 200 shell-free eggs/mouse in 0.1 ml of PBS. The mice were divided randomly into four groups, with ten animals per group. Group I served as the infected control group; each mouse was given 0.1 ml of distilled water with a few drops of 0.1% DSMO. Group II served as the praziquantel-treated group; PZQ was given at a dose of 25 mg/kg once, orally, according to Campos et al. (Reference Campos, Bressan and Evangelista1984), on day 21 post infection (pi). Groups III and IV served as the A. absinthium-treated groups; the crude aqueous extract was given at doses of 400 and 800 mg/kg, respectively, once, orally for three consecutive days, commencing on day 21 pi. Assessment of efficacies relied on reductions in the percentages of the egg count per gram of faeces (EPG) before and after treatment, and the worm burden at necropsy. The pre-treatment EPG count was performed on days 18–20 pi, whereas the post-treatment EGF count was performed on days 24–26 pi. The experiment was terminated by sacrificing all animals on day 30 pi.
Calculation of the reduction percentages of eggs per gram of faeces (EPG) count and the worm burden
The reduction percentage of the EPG count was calculated according to Iqbal et al. (Reference Iqbal, Lateef, Ashraf and Jabbar2004).
 $$\eqalign{&{\rm EPG\; count\; reduction\;} \left( {\rm \%} \right) \cr &\quad = \displaystyle{{{\rm Pre-treatment\; EPG\; count} - {\rm post-treatment\; EPG\; count}} \over {{\rm Pre-treatment\; EPG\; count}}} \cr &\qquad {\rm \; x\;}100.}$$
$$\eqalign{&{\rm EPG\; count\; reduction\;} \left( {\rm \%} \right) \cr &\quad = \displaystyle{{{\rm Pre-treatment\; EPG\; count} - {\rm post-treatment\; EPG\; count}} \over {{\rm Pre-treatment\; EPG\; count}}} \cr &\qquad {\rm \; x\;}100.}$$The worm burden reduction percentage was calculated according to Grzybek et al. (Reference Grzybek, Kukula-Koch, Strachecka, Jaworska, Phiri, Paleolog and Tomczuk2016).
where a = the mean number of worms recovered from the intestines of the infected and untreated (infection control group) mice, and b = the mean number of adult worms recovered from the treated mice.
Statistical analysis
All experimental data are presented as the mean ± standard deviation (SD) unless otherwise indicated. The data were analysed using the one-way analysis of variance (ANOVA) test followed by Tukey's post-hoc test to detect the statistical significance among the groups under study. Student's t-test was used to compare the pre-treatment and post-treatment data within each group. The results with P < 0.05 were considered significant.
Results
In vitro study
Worm paralysis and mortality
All tested concentrations of PZQ and the A. absinthium extract resulted in an initial spastic contraction of the adult worms within 10–30 s after exposure, followed by paralysis and then mortality of the treated worms at variable durations. Additionally, A. absinthium induced worm paralysis and death in a dose-dependent manner, and the difference between the A. absinthium concentrations used in groups III (1 mg/ml) and IV (5 mg/ml) was significant (P < 0.001) (table 1). Concerning the time of worm paralysis, a significant difference was found between both A. absinthium concentrations and PZQ. However, no significant difference was found in the time of worm death between A. absinthium at the 5 mg/ml concentration and PZQ (table 1).
Table 1. Times of paralysis and death of H. nana adult worms exposed to the tested drugs in vitro.
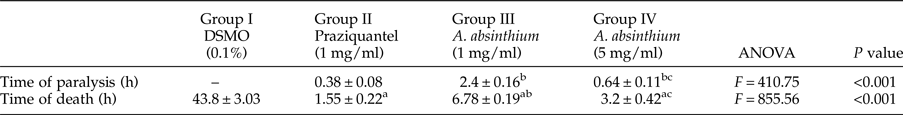
Time of death and time of paralysis were expressed as the mean ± SD; n = 5.
P < 0.05 was considered significant according to the post-hoc test: a, significant difference vs. group I; b, vs. group II and c, vs. group III.
Semi-thin and TEM ultrastructure morphological changes
The methylene blue-stained semi-thin sections of the control worms showed normal architecture of the tegumental area, with intact tegument, basal lamina and subtegumental tissues (fig. 1a). TEM revealed intact microtriches and basal lamina. Additionally, the subtegumental cells were rich in glycogen granules (fig. 1b). The tegument was rich in discoidal secretory bodies (fig. 1b, c). The muscular coat was formed with intact outer circular and inner longitudinal bundles (fig. 1b, d). The epithelial lining of the nephridial canal and its microvilli were intact (fig. 1e). Each intrauterine egg had an intact envelope and an intact oncosphere with hooks (fig. 1f).

Fig. 1. (a) A semi-thin section of an untreated control H. nana adult segment, showing the intact tegument (T), basal lamina (BL) and normal subtegumental architecture (ST), after staining with 1% methylene blue, 100× magnification. (b–f) TEM ultrastructures of untreated H. nana adults. (b) Tegumental area showing the intact brush border of the microtriches (MT) with the glycocalyx (GX), tegument (T) rich in discoidal secretory bodies (DSB, white arrows), basal lamina (BL), transverse muscle bundles (CM), and tegumental cells (TC) rich in electron-dense glycogen granules (G), 20,000× magnification. (c) Cut sections in an intact brush border of microtriches (MT). The tegument is rich in discoidal secretory bodies (DSB), 40,000×. (d) Normal longitudinal muscles (LM) and transverse muscle bundles (CM), 6000×. (e) Nephridial canal showing an intact epithelium and microvilli (M), 20,000×. (f) Intact egg showing the outer (O) and inner (I) envelopes, embryophore (E), and oncosphere (ON) with penetration gland (PG), hooks (HK) and oncospheral tegument (OT), 8000× magnification.
Following exposure to PZQ, the semi-thin sections showed massive destruction and vacuolization of the tegument and the subtegumental area (fig. 2a, b). Blebs of different sizes were also observed on the tegument (fig. 2b). TEM revealed a complete loss of the normal architecture with vacuolization (fig. 2c), and wide areas were filled with cell debris (fig. 2d).

Fig. 2. Segments of H. nana adult treated with praziquantel (1 mg/ml). (a, b) Semi-thin sections, showing: (a) destruction of the tegument (black arrows) and vacuolization (V) of the subtegumental area; and (b) blebs on the tegument and vacuolization (V) after methylene blue staining; 100× magnification. TEM micrographs of praziquantel-treated H. nana adult, showing: (c) areas of vacuolization (V), 6000×; (d) degenerated cells and areas full of cell debris, 8000× magnification.
Conversely, semi-thin sections of the H. nana adults treated with A. absinthium showed areas of sloughing of the tegument with exposure and/or destruction of the underlying basal lamina. Intense vacuolization (fig. 3a) and the accumulation of variable-sized lipid droplets in the subtegumental area were observed (fig. 3a, b). TEM showed that exposure to A. absinthium at the 1 mg/ml concentration resulted in the appearance of membrane-bound vesicles over the microtriches (fig. 4a), whereas the 5 mg/ml concentration resulted in the loss of the microtriches, interruption of the circular muscle layer and shrinkage of the subtegumental cells (fig. 4b). Additionally, an accumulation of lipid droplets in the muscle bundles was observed in a dose-dependent manner (fig. 4c, d). The 5 mg/ml concentration also resulted in the destruction of the epithelial lining of the nephridial canal with the loss of its lining microvilli (fig. 4e), and degeneration of the intrauterine eggs (fig. 4f).

Fig. 3. Semi-thin sections of H. nana adult segments treated with A. absinthium at a 5 mg/ml concentration, showing: (a) sloughed areas of the tegument (arrows), lipid (L) accumulation in the subtegument, areas of degeneration and destruction of the basal lamina (D) and intense vacuolization (V) after methylene blue staining, 100× magnification; (b) subtegumental area with accumulation of variably sized, yellowish lipid droplets after methylene blue staining, 400× magnification.

Fig. 4. TEM micrographs of A. absinthium-treated H. nana adults. (a) Tegumental area after treatment with 1 mg/ml showing microtriches (MT) with numerous membrane-bound vesicles, 40,000× magnification. (b) Tegumental area in an adult worm treated with 5 mg/ml showing loss of the microtriches (MT), with abundant dense secretory bodies (DSB), interruption of the circular muscle, shrunken subtegumental cells and widening of the areas occupied by connective tissue, 20,000×. (c) Accumulation of variably sized lipid droplets (L) in muscle bundles after treatment with 1 mg/ml of A. absinthium, 8000×. (d) Huge lipid droplet (L) accumulation in muscle bundles following treatment at a concentration of 5 mg/ml, 8000×. (e) Degeneration of the microvilli lining the nephridial canal (NC) following treatment with a concentration of 5 mg/ml, 12,000×. (f) Degenerated egg and the oncosphere (ON) lacking the hooks and penetration gland following treatment with a concentration of 5 mg/ml; O, outer envelope; I, inner envelope; E, embryophore; 6000× magnification.
At the level of the cellular structure, exposure to A. absinthium at the 1 mg/ml concentration resulted in the appearance of heterochromatin islands in the nucleus (fig. 5a, b) and numerous secretory vacuoles in the cytoplasm (fig. 5a). The 5 mg/ml concentration resulted in swelling of the nucleus, the appearance of a big nucleolus, shrinkage of the cytoplasm and the formation of many double-membrane vacuoles containing inclusion bodies. These vacuoles were observed adjacent to the rough endoplasmic reticulum (fig. 5c).

Fig. 5. (a, b) TEM micrographs of A. absinthium-treated (1 mg/ml) H. nana adults. (a) A subtegumental cell in between muscle bundles with glycogen granules (G) and a lipid droplet (L) starting to appear. The nucleus (N) contains a large electron-dense nucleolus (n) and heterochromatin islands (hc). The cytoplasm contains a rough endoplasmic reticulum (R), Golgi body (GL) and numerous secretory vacuoles (V). 20,000× magnification. (b) A cell showing shrunken cytoplasm and depletion of cellular organelles surrounded by intact muscle bundles (Mc) and areas rich in glycogen (G). Areas of glycogen depletion (D) were also observed. N, nucleus; n, nucleolus; hc, heterochromatin islands; 20,000× magnification. (c) Ultrastructure micrograph of a worm exposed to a 5 mg/ml concentration showing a myocyton with a swollen nucleus (N) with a big nucleolus (n). The cytoplasm contains a granular endoplasmic reticulum (R), many phagosomes (ph) containing inclusion bodies. The surrounding muscle bundles were infiltrated with variably sized lipid droplets (L), 10,000× magnification.
In vivo study
Reduction in the EPG and worm burden percentages
All of the tested drugs resulted in significant reductions in the EPG counts and worm burden (P < 0.001) (tables 2 and 3). Additionally, a significant difference was found between the pre- and post-treatment EPG counts within each group (table 2). Dose-dependent significant differences in reductions in the EPG (table 2) and worm burden (table 3) were found between A. absinthium-treated groups III (400 mg/kg) and IV (800 mg/kg). The results obtained with A. absinthium at the 800 mg/kg dose were comparable to the results obtained with PZQ, because the difference between groups II (PZQ) and IV (A. absinthium, 800 mg/kg) was not significant (P > 0.05) (tables 2 and 3).
Table 2. Pre- and post-treatment eggs per gram (EPG) and percentages of reduction among the different groups treated with the tested drugs.

EPG values are expressed as the mean ± SD; n = 10.
P < 0.05 was considered significant, according to the post-hoc test: a, significant difference vs. group I; b, vs. group II and c, vs. group III.
* Indicates a significant difference between the pre- and post-treatment EPG within each group according to Student's t-test.
Table 3. Worm burden and its percentage reduction among the different groups treated with the tested drugs.

Worm numbers per mouse were expressed as the mean ± SD; n = 10.
P < 0.05 was considered significant according to the post-hoc test: a, significant difference vs. group I; b, vs. group II and c, vs. group III.
Discussion
The present study aimed to evaluate the therapeutic effects of the crude aqueous extract of the traditionally well-known medicinal herb A. absinthium against the common intestinal tapeworm H. nana, in comparison with the drug of choice (praziquantel), in controlled in vitro and in vivo experiments. The results of this study revealed promising anticestodal effects of A. absinthium against adult H. nana, both in vitro and in vivo. These results were in accordance with previous studies utilizing A. absinthium herbal extracts against different parasitic diseases and recorded similar effects. For example, Caner et al. (Reference Caner, Döşkaya, Değirmenci, Can, Baykan, Uner, Başdemir, Zeybek and Gürüz2008) reported that the methanolic extract was effective against enteral and parenteral phases of experimental trichinellosis. Additionally, Ferreira et al. (Reference Ferreira, Peaden and Keiser2011) documented that the ethanolic extract, at a 2 mg/ml concentration, was effective against S. mansoni and F. hepatica adults in vitro. Tariq et al. (Reference Tariq, Chishti, Ahmad and Shawl2009) reported that both the ethanolic and aqueous extracts of A. absinthium showed anthelmintic activity against Haemonchus contortus adult worms in vivo and in vitro. However, the authors found that the ethanolic extract was more efficient than the aqueous extract.
Generally, alcoholic extracts of medicinal plants show better anthelmintic properties than aqueous extracts (Eguale et al., Reference Eguale, Tilahun, Debella, Feleke and Makonnene2007). However, use of the A. absinthium aqueous extract at the 5 mg/ml concentration in vitro and the 800 mg/kg dose for three consecutive days in vivo produced results close to those obtained with PZQ. Comparing these groups with PZQ revealed no significant differences (P > 0.05) regarding the time of worm death and the reduction percentages of EPG and the worm burden. In contrast to the present study, the ethanolic extract of A. absinthium was found to be less effective than the reference drugs against intestinal coccidiosis in goats (Iqbal et al., Reference Iqbal, Tariq, Wazir and Singh2013). One explanation for this discrepancy could be that the antiparasitic activities of medicinal plants or their products depend not only on the nature of the extract (aqueous or alcoholic) but also on the plant composition, which varies from country to country (Orav et al., Reference Orav, Raal, Arak, Murisepp and Kailas2006), the targeted parasitic species and the concentrations or doses used (Ferreira et al., Reference Ferreira, Peaden and Keiser2011). In the present study, the effectiveness of A. absinthium against H. nana was dose-dependent, which was in accordance with previous studies using different types of plant extracts against many parasites (Bashtar et al., Reference Bashtar, Hassanein, Abdel-Ghaffar, Al-Rasheid, Hassan, Mehlhorn, Al-Mahdi, Morsy and Al-Ghamdi2011; Kundu et al., Reference Kundu, Roy and Lyndem2012; Deori & Yadav, Reference Deori and Yadav2016).
Generally, cestodes lack a digestive system. Therefore, all nutritive and gaseous exchanges must occur through their tegument and the microtriches, which are considered vital structures responsible for nutrition, immunoprotection and osmoregulation (Roy et al., Reference Roy, Dasgupta and Tandon2008). With respect to the morphological changes induced by the tested drugs in the teguments of the treated worms, we observed an obvious loss of the normal architecture in the worms exposed to all the drugs tested in vitro.
In this study, exposure to PZQ resulted in a complete loss of the normal architecture, with intense vacuolization and cellular degeneration. To explain how these changes were induced by PZQ, Prichard et al. (Reference Prichard, Bachmann, Hutchinson and Kohler1982) suggested the occurrence of a sustained release of endogenous calcium with a subsequent increase in the calcium concentration in the cytoplasm, which might result in a spastic contraction of the PZQ-treated worms, followed by vacuolization of their teguments. The increase in the size of these vacuoles results in their protrusion above the surface, to become visible as blebs. These blebs finally burst, resulting in leakage of glucose and amino acids (Andrews et al., Reference Andrews, Thomas, Pohlke and Seubert1983). Continuous leakage would result in intense cellular destruction and worm death. Therefore, the results of the present study were in accordance with many studies that considered PZQ the anticestodal drug of choice (Kundu et al., Reference Kundu, Roy and Lyndem2012; Deori & Yadav, Reference Deori and Yadav2016; Giri & Roy, Reference Giri and Roy2016).
Conversely, exposure to A. absinthium at a 1 mg/ml concentration resulted in the formation of membrane-bound vesicles over the microtriches and the accumulation of variably sized lipid droplets in the muscle bundles. At a 5 mg/ml concentration, A. absinthium resulted in a complete loss of the microtriches, interruption of the circular muscle, glycogen depletion, shrinkage of the subtegumental cells, accumulation of large lipid droplets, destruction of the epithelial lining of the nephridial canal and degeneration of the intrauterine eggs.
Alteration or loss of the microtriche brush border would affect the nutrient absorption process, and thus the worms would begin to consume their glycogen stores. This scenario could explain the occurrence of glycogen depletion upon exposure to the A. absinthium crude extract in this study. O'Neill et al. (Reference O'Neill, Johnston, Halferty, Brennan, Keiser and Fairweather2009) and Dasgupta et al. (Reference Dasgupta, Roy and Tandon2010) claimed that glycogen depletion together with mitochondrial dysfunction, which occurs as a result of stressful conditions, such as exposure to drugs (Pérez-Serrano et al., Reference Pérez-Serrano, Denegri, Casado and Rodriguez-Caabeiro1997), would result in impairment of the tegument. In the present study, not only was the nutrient uptake affected but the excretory system was also disturbed, which would result in an accumulation of waste products. Thus, the worms exposed to A. absinthium were most likely pushed into a vicious circle of depletion and damage. However, these worms would try to repair the damaged tegument by activation of cellular organelles to overcome the rapid turnover of the tegument (McCaigue et al., Reference McCaigue, Halton and Hopkins1986). These trials to repair the damaged tegument could explain the formation of membrane-bound vesicles over the microtriches of the A. absinthium-treated worms, the appearance of an active Golgi apparatus and the numerous cytoplasmic secretory vacuoles (fig. 5a).
In addition to the stressful conditions, the mitochondria could also be affected by artemisinins, which were reported to cause inhibition of electron transfer and oxidative phosphorylation (Li et al., Reference Li, Mo, Shen, Sun, Wang, Lu, Gitschier and Zhou.2005), collapse of the mitochondrial membrane potential and triggering of cytochrome c release from the mitochondria into the cytoplasm, with final activation of caspase-3-mediated apoptosis (Jia et al., Reference Jia, Qin, Zhang, Guo, Wang, Yue and Qian2016). In the present study, TEM revealed some morphological signs of apoptosis in the A. absinthium-treated worms (fig. 5a, b), such as condensation and clumping of chromatin into heterochromatin islands, as described by Bashtar et al. (Reference Bashtar, Hassanein, Abdel-Ghaffar, Al-Rasheid, Hassan, Mehlhorn, Al-Mahdi, Morsy and Al-Ghamdi2011) and Roy et al. (Reference Roy, Dasgupta and Giri2012) in parasites exposed to different plant extracts.
Accordingly, double-membrane phagocytic vacuoles containing inclusion bodies (most probably the destroyed organelles) were found upon exposure to the 5 mg/ml concentration of A. absinthium in this study (fig. 5c). These vacuoles were found in a close contact with the rough endoplasmic reticulum and thus were identified as autophagosomes according to Česen et al. (Reference Česen, Pegan, Spes and Turk2012). The appearance of these autophagosomes is the morphological hallmark of autophagy (a self-eating process) (Eskelinen et al., Reference Eskelinen, Reggiori, Baba, Kovcs and Seglen2011). This process usually occurs as a survival mechanism in cells exposed to stressful conditions such as starvation and pharmacological insults (Loos et al., Reference Loos, Caparros, Nicolao, Denegri and Cumino2014). Through autophagy, denatured proteins and damaged organelles are degraded into amino acids to be recycled in the synthesis of the essential proteins needed for the survival of these struggling cells (Xie & Klionsky, Reference Xie and Klionsky2007). Thus, the appearance of these autophagosomes in the cells of the worms exposed to A. absinthium suggested an attempt to salvage any available nutrients to remain alive. This finding was in accordance with Loos et al. (Reference Loos, Caparros, Nicolao, Denegri and Cumino2014), who reported the occurrence of autophagy in the larval stages of Echinococcus granulosus upon exposure to pharmacological stress.
In the present study, accumulation of lipid droplets in the muscle bundles was seen with administration of A. absinthium in a dose-dependent manner (Fig. 4c, d). McCaigue et al. (Reference McCaigue, Halton and Hopkins1986) also recorded lipid deposition in the immune-induced dark areas of H. diminuta. The latter authors explained this finding due to the lack of balance between the intake, use and excretion of lipids. They assumed that the normal lipid absorption pathway and its use in phospholipid synthesis would be stimulated and integrated into the cell membranes for tissue repair. Additionally, inhibition of lipid metabolism and excretion, which are energy-dependent processes, was expected to occur as a result of the impaired functions of the mitochondria and depletion of the glycogen store. Collectively, all of these factors would explain the occurrence of lipid accumulation in the muscle bundles of the A. absinthium-treated worms.
In the light of the above findings, the A. absinthium crude aqueous extract showed promising effects against adult H. nana, both in vitro and in vivo, in a dose-dependent manner. These results were comparable to the results induced by the reference drug praziquantel. In vitro, A. absinthium was able to induce worm paralysis and death. TEM examination of the treated worms revealed morphological changes, such as tegumental damage, lipid accumulation, and destruction of the nephridial canal and the intrauterine eggs. Additionally, signs of apoptosis and autophagy were observed, which could be ascribed to the impairment of nutrient uptake due to the tegumental damage caused by A. absinthium. Therefore, the damaged worms started to consume their glycogen stores. Lipid accumulation occurred due to inhibition of lipid metabolism and excretion, which are energy-dependent processes. The continuous exposure to stress and the accumulation of waste products most likely triggered the apoptosis process. However, these affected worms would not surrender to death without trying to remain alive through the autophagy (self-eating) process. Moreover, results of the in vivo study supported those obtained in the in vitro study, because A. absinthium induced significant reductions in EPG and the worm burden. Collectively, these results demonstrate the anticestodal efficacy of A. absinthium against H. nana.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
All of the experimental animal studies were conducted in accordance with the valid international guidelines after approval by the Scientific Research Ethical Committee, Faculty of Medicine, Menoufia University.









