INTRODUCTION
Nematodes of the genus Trichinella are the causative agents of trichinellosis, a zoonotic disease with clinical symptoms in humans ranging from mild to fatal. Trichinella spp. also occur in many carnivorous and omnivorous animal species, but animal infections do not lead to clinical signs [Reference Pozio1, Reference Kociecka2]. Transmission of infection occurs via the intake of meat containing infective larvae [Reference Pozio3, Reference Murrell and Pozio4]. Appropriate heat or freezing treatment are effective to inactivate larvae [Reference Gajadhar5], and therefore human infections are caused by the consumption of raw or undercooked meat. Wild boar meat, horse meat and pork are the main sources for human infection in Europe [6].
Testing of all slaughtered pigs for the presence of larvae is mandatory in the European Union (EU) and Switzerland to prevent human disease [7]. Despite routine testing at pig slaughter in Switzerland since 2001, no larvae have ever been detected [8]. A recent study also failed to detect anti-Trichinella antibodies in domestic pigs [Reference Schuppers9]. Presence of antibodies without direct detection of the parasite would be an indicator for the presence of low-grade Trichinella infections that are not detectable by routine artificial digestion.
EU Regulation 2075/2005 requires that 1 g (finishing pigs) or 2 g (adult pigs) of diaphragm tissue per pig are tested using the routine artificial digestion method during meat inspection. The sensitivity of this method depends on the larval density of the positive samples. Above a larval density of 3–5 larvae per gram (LPG), a sensitivity of 100% was achieved, but below 1 LPG the sensitivity dropped to 40% [Reference Forbes and Gajadhar10]. Because ∼15–20% of naturally infected pigs harboured larval densities of <1 LPG [Reference Pozio and Rossi11], infected pigs may not be detected reliably by this method. Despite the large financial efforts involved in testing of all slaughtered pigs during meat inspection, this surveillance is not adequate to prevent human consumption of pork containing low larval densities. However, if surveillance continues over several years without detecting any infected pigs, these surveillance data can be used to demonstrate that the domestic pig population of a country is free from Trichinella infection [Reference Martin, Cameron and Greiner12, Reference Martin13].
Instead of applying the routine artificial digestion method to all pigs during meat inspection, a risk-based surveillance programme could be developed that targets high-risk pigs and uses a diagnostic test protocol with a high sensitivity. Targeted sampling of high-risk pigs increases the confidence that infection is truly absent when all samples test negative, whereas a diagnostic test system with a high sensitivity increases the probability of detecting infection if present. Such a risk-based surveillance programme should provide at least an equivalent level of consumer protection as the current meat inspection programme.
The probability of infection of a pig depends on age and housing conditions. In older pigs this probability is higher due to the cumulative effect of longer lives [Reference Pozio and Rossi11]. Housing conditions determine access to potentially infected wildlife (carrion) and feeding of slaughter and kitchen waste, both of which are important routes of infection [Reference Pozio3, Reference Pozio14]. Swiss pig production meets high hygiene standards, thus reducing the importance of feeding of waste materials, but T. britovi is known to occur in Swiss wildlife [Reference Gottstein15, Reference Frey16]. Domestic pigs with outdoor access therefore have a higher probability of being exposed to Trichinella spp. than pigs entirely raised indoors.
The first goal of this study was to evaluate the probability that the Swiss slaughter pig population is truly free from Trichinella larvae based on the data from the current meat inspection programme, and to model the future probability of freedom if this surveillance is continued in its current format. The second goal was to develop a risk-based surveillance programme for Trichinella spp. in domestic pigs that provides an equivalent probability of freedom from infection in the Swiss pig population.
MATERIALS AND METHODS
Target population
The target population for this study consisted of all slaughtered pigs in Switzerland, the unit of surveillance being one slaughtered pig. The time period for analysis was 1 year.
Model
Disease freedom is usually defined as a certain level of confidence that the true prevalence is below a specified design prevalence [Reference More17]. Freedom from Trichinella infection of the target population can be demonstrated when all pigs tested within the surveillance programme have negative test results. The achieved probability of freedom depends on the number of tested pigs and the test characteristics of the diagnostic test. The probability of freedom increases when all test results are negative for multiple surveillance time periods. A Bayesian approach [Reference Martin, Cameron and Greiner12, Reference Martin13] was used to calculate the probability of freedom using data from multiple surveillance time periods. The model depends on several parameters:
• the design prevalence, P*;
• the sensitivity of the surveillance system, SSe;
• the probability of introduction, PIntro;
At the beginning of each time period tp, a certain prior probability exists that the target population is infected. This probability is reflected by PriorPinftp. At the end of tp it is possible to calculate the posterior probability of freedom PostPfreetp using Bayes' theorem assuming perfect specificity of the surveillance system [Reference Martin, Cameron and Greiner12, Reference Martin13]:
Two alternative designs were calculated and compared. In the first design, the surveillance programme was based on the use of the routine artificial digestion test at slaughter. Slaughtered pigs were tested without consideration of their relative risk (RR) of infection, so no risk groups were included in the first design. Data from the routine artificial digestion test were used that were available for the period 2001–2007. Data from 2007 were extrapolated until 2015 to obtain a 15-year surveillance period, assuming the surveillance system would not change from 2008 to 2015, and no positive results would be recorded. This assumption was considered reasonable, because the data from 2007 reflected a full-scale testing programme in Switzerland and the size of the slaughter pig population has remained stable over the last 7 years.
In the second design, a risk-based, serological surveillance programme was considered. An ELISA was used as screening test, and a Western Blot assay (WB) was used as a confirmatory test for any ELISA-positive samples [Reference Schuppers9, Reference Frey18]. The target population was divided into different risk groups depending on age and housing conditions, and groups with a higher risk were sampled more intensively than groups with a lower risk. The risk-based surveillance programme was also modelled for a 15-year period starting in 2010, directly following 9 years of surveillance in design 1.
The model was built as a scenario tree with multiple branches (Table 1). First, the total pig population was stratified according to the risk factors age and housing condition. Then, the probability of infection for a randomly selected pig in each of the different strata was determined. Clustering at herd level was not included in the model, because trichinellosis is not a contagious disease and the mere presence of an infected pig therefore does not increase the probability of infection for nearby pigs.
Table 1. Scenario tree structure for risk-based serological Trichinella surveillance in domestic pigs in Switzerland, assuming perfect specificity of the surveillance system

For infected pigs, the diagnostic test system could either correctly confirm this status (outcome=positive), or fail to detect the infected pig (outcome=negative). The specificity of the surveillance system was considered to be 100%. The assumption of perfect specificity is common for programmes demonstrating freedom [Reference More17, Reference Alban19], because a positive finding after confirmatory investigations would imply the loss of the ‘free status’ and the surveillance to demonstrate freedom would be replaced by surveillance to regain the ‘free status’. Moreover, the specificity of the WB was 100% or very near [Reference Frey18, Reference Frey20, Reference Nöckler21].
The models were created in Microsoft Excel with the add-in @Risk (Palisade Inc., USA). The models were stochastic models with appropriate probability distributions as inputs, and were run with 10 000 iterations. A regression analysis was conducted in @Risk to identify the input parameters with the greatest influence on the model outcome (probability of freedom from infection).
Slaughter pig population
In the period 2001–2007, 2·6–2·8 million pigs were slaughtered annually in Switzerland (Table 2). Routine artificial digestion tests had been implemented voluntarily since 2001 and were made compulsory in 2007 [22], although an exception is made for small-scale slaughterhouses that only market their products locally. The results of the routine artificial digestion tests are presented in Table 2. For the risk-based surveillance programme a slaughter pig population of 2·7 million pigs per year was assumed. The slaughter statistics did not allow differentiation between age categories or housing conditions. Therefore, these data had to be derived from other sources.
Table 2. Number of pigs slaughtered and tested for Trichinella spp. in Switzerland, 2001–2007
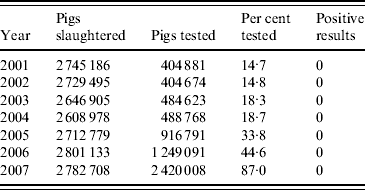
Source: Federal Veterinary Office, Swiss Zoonoses Reports 2005–2008 (http://www.bvet.admin.ch/dokumentation/00327/index.html?lang=en). Accessed 23 July 2009.
In 2006, the adult pig population was estimated at 155 000 animals [23]. Assuming an annual replacement rate of ∼40%, around 62 000 adult pigs were slaughtered in 2006, representing 2·3% of the total slaughter pig population. This percentage was similar to the numbers presented for Denmark [Reference Alban19]. The proportion of slaughtered finishing pigs (PrP finish) was thus modelled as Pert(0·97, 0·98, 0·99) to allow for small variations in the actual proportions and the proportion of slaughtered adult pigs (PrP adult) as 1 – PrP finish.
A large proportion of the Swiss pig population is kept in production systems with access to outdoor areas. According to the annual report of the Swiss Federal Office of Agriculture [24], 61% of all finishing pigs and 58% of all adult pigs have access to outdoor areas. In the majority of cases, these outdoor areas consist of small, confined areas with concrete floors (housing condition: outdoor). Rarely, pigs are kept on pasture under extensive conditions (free-range), but no estimates for the number of pigs in this category were available. Using expert opinion, it was estimated that 2% of all finishing pigs and 1% of all adult pigs fell in the free-range category. The remaining pigs (37% of all finishing pigs and 41% of all adult pigs) were assumed to be produced under intensive conditions without outdoor access (indoor). To account for uncertainty around these point estimates, the proportions of indoor finishing pigs (PrP finish,in) and indoor adult pigs (PrP adult,in) were modelled as Pert(0·32, 0·37, 0·42) and Pert(0·36, 0·41, 0·46), respectively. The proportion of outdoor finishing pigs (PrP finish,out) was modelled as Pert(0·56, 0·61, 0·66) and of outdoor adult pigs (PrP adult,out) as Pert(0·53, 0·58, 0·63). The proportion of free-range finishing pigs was then calculated as 1−(PrP finish,in+PrP finish,out) and of free-range adult pigs as 1−(PrP adult,in+PrP adult,out).
Design prevalence and effective probability of infection
P* was set at 0·0001%, as defined by EU Regulation 2075/2005. Although P* applied to the whole target population, the effective probability of infection (EPI) differed between the different risk groups. However, the average EPI of all pigs still equalled P*.
The EPI for a pig is derived from the RRs associated with the applicable levels of each of the risk factors specified, i.e. age and housing condition. For each risk factor, RR is the risk of infection in its risk category relative to the risk in the lowest risk category for that risk factor. No cases of Trichinella-positive pigs have been reported in Switzerland, and also in other Western European countries there is a lack of data to reliably determine the RR of individual pigs in the different risk groups.
The RR of adult pigs in comparison to finishing pigs is derived from the longer lifespan and thus the increased probability of infection at some time during life. Finishing pigs are slaughtered at around age 6 months, and the average breeding sow is slaughtered at around 3·5 years of age (assuming five litters per sow). If the probability of infection during life increased linearly, at slaughter a breeding sow would have a seven times higher probability of having acquired an infection than a finishing pig. To account for uncertainty around this assumption, two different RRs for adult pigs in comparison to finishing pigs were used:
The RR of pigs raised under outdoor or free-range housing conditions in comparison to pigs under indoor housing conditions is determined by the differences in biosecurity of these housing conditions and thus the probability that pigs in these different housing conditions have contact with infected wildlife or contaminated kitchen or slaughter waste. No estimates for RRs were available, therefore two different increments were selected. First, it was assumed that the RR increased by a factor of 5 between housing conditions (RRoutdoor=5 and RRfree-range=25). Second, it was assumed that the RR increased by a factor of 10 between housing conditions (RRoutdoor=10 and RRfree-range=100).
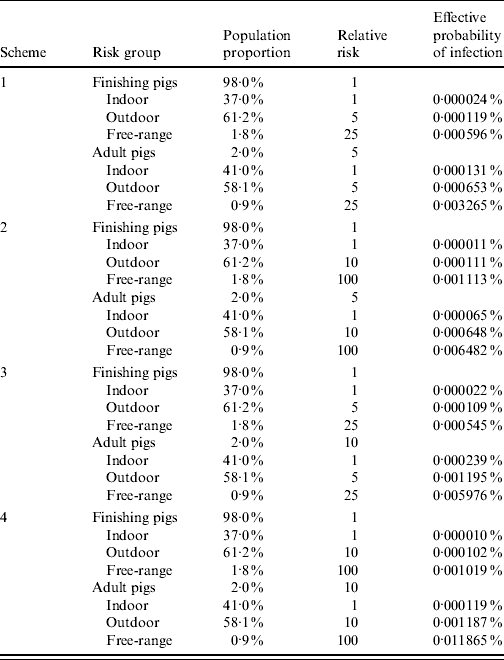
Combining these two risk factors (age and housing condition) into a matrix, four schemes were developed (Table 3). Relative risks were then adjusted to give adjusted risks (ARs), such that the average AR over the target population was 1 [Reference Martin, Cameron and Greiner12, Reference Martin13]. For age:
in which the target population was divided into L different age categories, and PrP l was the proportion of animals in the target population belonging to age group l. This process was repeated for the risk factor housing condition using the appropriate conditional proportions. Then [Reference Martin, Cameron and Greiner12, Reference Martin13]:
where m denotes categories of housing condition.
Table 3. Relative risks of Trichinella infection associated with age and housing condition in four combinations (schemes), and adjusted prevalence (effective probability of infection) for each risk group separately. Design prevalence for whole population, P*=0·0001%
Diagnostic tests and the sensitivity of the surveillance system
For the routine artificial digestion test, samples of up to 100 pigs can be pooled. It was demonstrated that the sensitivity of a pooled assay with 100 samples did not exceed 40% in case of larval densities <1 LPG [Reference Forbes and Gajadhar10], a situation that occurs in 15–20% of the pigs infected under field conditions [Reference Pozio and Rossi11]. As a conservative approach for design 1, it was therefore assumed that the sensitivity of the routine artificial digestion test (Se AD) was 40% and it was modelled as Pert(0·35, 0·40, 0·45) [Reference Alban19].
For design 2, an ELISA and WB were used as screening and confirmatory tests, respectively. Various studies evaluated the sensitivity of the ELISA (Se ELISA) and reported values from 72·7% to 99·2% [Reference Frey20, Reference Murrell25–Reference Nöckler28]. Se ELISA was therefore modelled as Pert(0·60, 0·95, 1). The WB was recently validated with reported sensitivities of 95·8–98·1% [Reference Frey18, Reference Frey20, Reference Nöckler21]. The sensitivity of the WB (Se WB) was therefore modelled as Pert(0·90, 0·98, 1).
The SSe is an estimate of the probability that the surveillance system detects infection in the target population if the prevalence exceeds P*. SSe is calculated as [Reference Martin, Cameron and Greiner12, Reference Martin13]:
in which Se u is the probability that a randomly sampled animal (unit) is both infected and detected and N is the total number of animals in the surveillance system. Equation (4) assumes independence of animals with regard to the probabilities of being infected and detected. In design 1, no risk groups were included and Se u was therefore calculated as:
In design 2, an animal in any of the risk groups can give a positive outcome, so Se u was calculated as:
in which PrSSC l,m was the proportion of pigs processed that belonged to the lth age stratum and the mth housing condition stratum.
Probability of introduction
T. britovi is present in Swiss wildlife [Reference Frey16], and constitutes a risk for introduction of infection into the domestic pig population. However, no records of infected domestic pigs exist in Switzerland, and PIntro therefore cannot be derived directly. Alban et al. [Reference Alban19] conservatively determined PIntro for the Danish domestic pig population as 1 divided by the time since the last outbreak, resulting in 1/76. Since this was a conservative estimate, we considered it valid to use a similar PIntro for the Swiss pig population. We modelled PIntro as a Beta distribution with 0 introductions in 75 years [Beta(1, 76)], resulting in a median annual PIntro of 0·91% (95% probability interval 0·03–4·7). Taking into account the higher proportion of pigs having access to outdoor areas in Switzerland and the presence of T. britovi in wildlife, we also modelled PIntro as a Beta distribution with 0 introductions in 50 years [Beta(1, 51)], resulting in a median annual PIntro of 1·3% (0·05–7·0).
RESULTS
Design 1: traditional Trichinella surveillance
The SSe increased gradually from 14·95% in 2001 to 62·02% in 2007, because the sample size increased annually during this period. From 2008–2015 the SSe remained equal to the SSe in 2007, because the number of pigs tested was kept constant. The PriorPinf2001 was set at 50%, because no other information was available. Depending on the selected PIntro, Switzerland could demonstrate freedom from Trichinella infection in domestic pigs with 95% confidence by the end of 2010 or 2012 (Fig. 1).
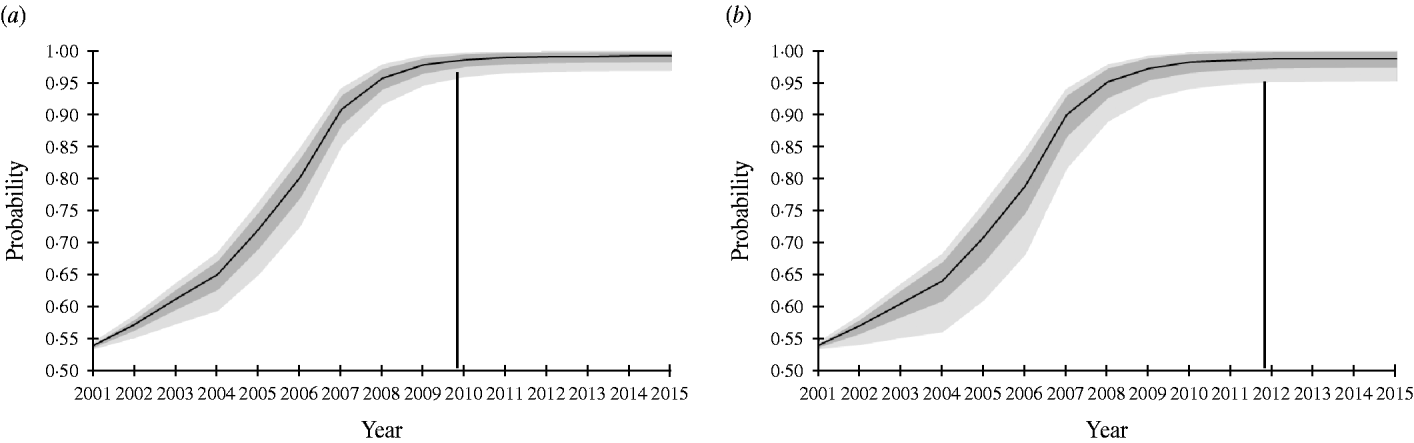
Fig. 1. Probability of freedom from Trichinella spp. infection of the Swiss slaughter pig population at a design prevalence of 0·0001% achieved at the end of each surveillance year using routine artificial digestion without considering risk groups in the pig population. Vertical line indicates year at which end the probability of freedom exceeds 95%, as expressed conservatively by the lower limit of the 95% confidence interval. Black line represents mean. Dark grey area, ±1 standard deviation; light grey area, 95% confidence interval. (a) Probability of introduction (PIntro)=Beta(1, 76); (b) PIntro=Beta(1, 51).
The input parameters Se AD and PIntro had the largest influence on the model, although their relative importance changed over time. For example, when PIntro=Beta(1, 76), the regression coefficients of Se AD and PIntro changed from 0·64 and −0·77, respectively after year 2 to 0·12 and −0·99, respectively after year 15. Regression coefficients were very similar when PIntro=Beta(1, 51).
Design 2: risk-based Trichinella surveillance
In risk-based surveillance, freedom from infection must also be demonstrated with at least 95% probability. The PriorPinf2010 (the year in which the risk-based surveillance programme started) was calculated using the PostPinf2009 of design 1. This was considered appropriate, because the risk-based surveillance programme started immediately after the completion of the traditional surveillance in 2009. The sampling was targeted towards the higher risk groups, and included almost all adult pigs, almost all free-ranging finishing pigs, a large number of outdoor finishing pigs and a small number of indoor finishing pigs. The minimum sample size was determined by increasing the sample size by steps of 10 000 samples until freedom from infection was demonstrated (Table 4). For PIntro=Beta(1, 76), the required sample sizes ranged from 120 000 (scheme 4) to 360 000 (scheme 1). For PIntro=Beta(1, 51), the required sample sizes ranged from 260 000 (scheme 4) to 620 000 (scheme 1). Figure 2 shows the probability of freedom from infection achieved by the risk-based surveillance programme from 2010 to 2024 under scheme 1.
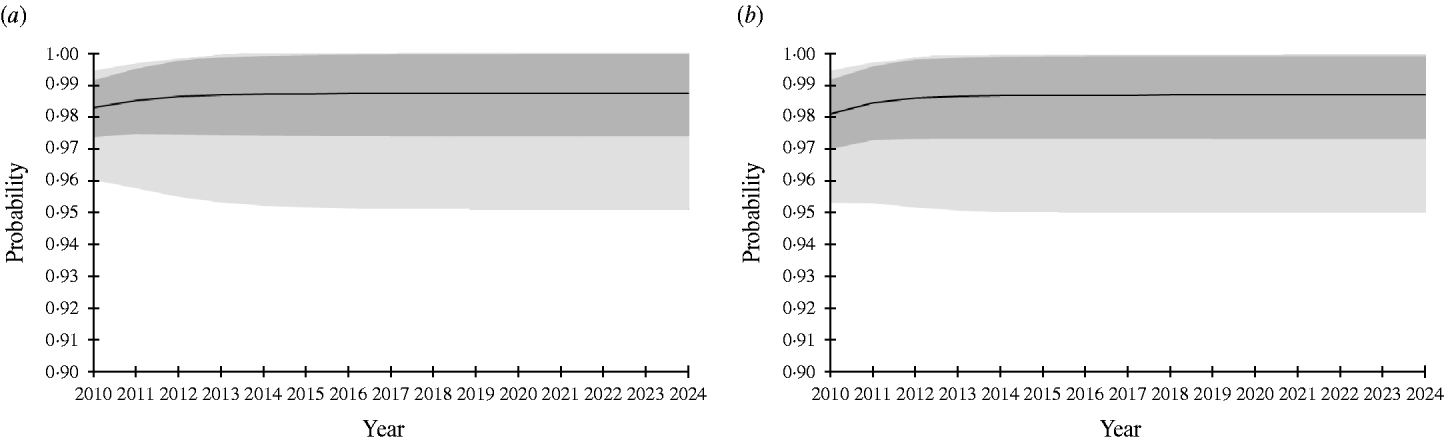
Fig. 2. Probability of freedom from Trichinella spp. infection of the Swiss slaughter pig population at a design prevalence of 0·0001% achieved at the end of each surveillance year using ELISA and Western Blot assay and considering risk groups in the pig population. Black line represents mean. Dark grey area, ±1 standard deviation; light grey area, 95% confidence interval. (a) Probability of introduction (PIntro)=Beta(1, 76); (b) PIntro=Beta(1, 51).
Table 4. Minimum required sample size to demonstrate freedom from Trichinella infection of the Swiss domestic pig population with at least 95% confidence after 15 years of negative risk-based serological surveillance
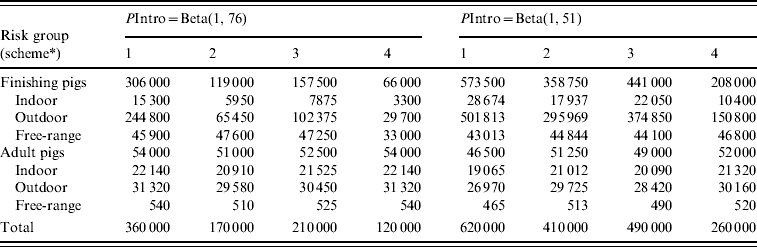
PIntro, Probability of introduction.
* Schemes 1–4 each have a different combination of relative risks for the risk factor age (finishing pigs vs. adult pigs) and housing conditions (indoor vs. outdoor vs. free range).
The SSe differed for each of the four schemes due to different sample sizes, and was also influenced indirectly by PIntro, because a higher PIntro resulted in higher sample sizes. After the required sample sizes had been established, the SSe was determined. For PIntro=Beta(1, 76), the median SSe of schemes 1–4 varied between 51·3–52·4%. For PIntro=Beta(1, 51), the median SSe of schemes 1–4 varied between 61·1 and 61·3%.
After 1 year of surveillance, the model was mainly influenced by four input parameters. For PIntro=(1, 76), in scheme 1 the regression coefficients were PIntrodesign2=−0·72, PIntrodesign1=−0·60, Se AD=0·29 and Se ELISA=0·10. After 15 years, two main input parameters remained: PIntrodesign2=−0·98 and Se ELISA=0·11. Regression coefficients were very similar for the other schemes.
DISCUSSION
This study demonstrated that surveillance by routine artificial digestion test is not capable of demonstrating freedom from Trichinella infection in the domestic pig population at the desired level of confidence based on data from a single year in Switzerland. To achieve this, a much larger slaughter pig population would be required than is available in Switzerland. Freedom from Trichinella infection by traditional surveillance can only be demonstrated when historical data are incorporated. The method developed by Martin et al. [Reference Martin, Cameron and Greiner12, Reference Martin13] allowed this, by assuming that the posterior probability of freedom achieved in year t – 1 could be used to derive the prior probability of freedom in year t. However, even when historical data were incorporated, freedom from infection could no longer be demonstrated when the sample size was reduced to 1 million pigs per year (data not shown). Therefore, Switzerland would need to continue testing almost all slaughtered pigs at slaughter if routine meat inspection alone was used to demonstrate freedom from infection.
The sample size could be reduced significantly when serological tests were used and the different risk groups within the pig population were taken into account. Depending on the scheme selected, the annual sample size was reduced by at least a factor of 4 without a loss in the probability of freedom from infection. Further, freedom from infection was already demonstrated after 1 year of risk-based serological surveillance.
Alban et al. [Reference Alban19] developed a risk-based surveillance model for Trichinella spp. in domestic pigs in Denmark. In this model all adult pigs and all finishing pigs with outdoor access were sampled, whereas finishing pigs from indoor housing systems were not sampled. However, this model used the routine artificial digestion test instead of serology. Serology has two advantages over the routine artificial digestion test. First, especially with low larval densities the diagnostic sensitivity of ELISA and WB is higher than of routine artificial digestion [Reference Forbes and Gajadhar10, Reference Frey20, Reference Murrell25–Reference Nöckler28]. Second, the number of larvae triggering a detectable antibody response is much lower than the number of larvae that can be detected reliably by routine artificial digestion test [Reference Gamble29], leading to a higher analytical sensitivity of serology. Thus, the probability of detecting low-grade infections in pigs increases when serology is used, which additionally supports claims of freedom from infection when all samples are negative.
In the present calculations, a positive outcome was defined as detection of antibodies by both ELISA and WB. Detection of larvae was not included, which is usually considered a reference for determining the infection status of a pig [Reference Gajadhar5, Reference Gamble30]. However, presence of antibodies indicates that the tested pig has previously been in contact with Trichinella spp. False-positive results of the ELISA were excluded by the use of a WB. The combination of both tests was previously shown to have a specificity of at least 99·8–99·9% [Reference Frey18, Reference Frey20]. In case antibodies were demonstrated by WB, investigations should be initiated on the farm of origin to assess the opportunities for exposure of pigs to Trichinella spp.
The sensitivity analysis showed that PIntro was the most important input variable for the model. Very limited data were available to estimate PIntro. The first approach was to use a similar value as used by Alban et al. [Reference Alban19], who already discussed that this value was a conservative estimate. However, the situation in Denmark is different from Switzerland. T. britovi is known to occur regularly in Swiss wildlife [Reference Gottstein15, Reference Frey16], whereas Trichinella spp. is rare in Danish wildlife [Reference Enemark31]. Moreover, outdoor housing of pigs is much more common in Switzerland than in Denmark [Reference Alban19, 24]. Therefore, in a second approach an even more conservative PIntro was used to take these two differences into account. Further, the sampling in the risk-based surveillance model was heavily targeted towards pigs in the higher risk groups. Despite the increased PIntro, freedom from infection could still be demonstrated in the Swiss domestic pig population.
There are very few data about the RRs of pigs acquiring a Trichinella infection. It is generally accepted that pigs with outdoor access as well as adult pigs have a higher probability of infection, but this probability was never quantified. Ribicich et al. [Reference Ribicich32] determined that Trichinella infections occurred in pigs raised outdoor but not in pigs raised in confinement or partial confinement, however a RR could not be determined. In other studies infections were also detected more frequently in pigs with outdoor access than in pigs in indoor housing systems [Reference van der Giessen33, Reference Gebreyes34]; however, RRs were not calculated. Alban et al. [Reference Alban19] arbitrarily defined four scenarios with different RRs for the high-risk group, ranging from 5·5 to 69. In this study four different schemes for the RR were also used to compensate for the uncertainty around the estimates. Scheme 1 was considered to be the most conservative scheme, because the RRs were minimal. This scheme therefore also leads to the highest required sample sizes.
The ability to identify and trace pigs of the different risk groups clearly is a crucial element for the successful implementation of a risk-based surveillance system. Currently, such identification and traceability is only possible in Switzerland with an unjustifiably high input of resources. Production labels (e.g. organic production) are poor indicators for the actual pig housing conditions, because farmers may voluntarily exceed the minimum label requirements. Improvement of the pig identification system should be considered before a change to a risk-based surveillance for Trichinella spp. is feasible in Switzerland.
In conclusion, this study demonstrated that risk-based serological Trichinella surveillance is able to achieve a probability of freedom from infection equivalent to routine artificial digestion, while the required sample size can be reduced by at least a factor of 4.
ACKNOWLEDGEMENTS
The authors thank Tony Martin for his useful comments on this manuscript. This research was funded by the Swiss Federal Veterinary Office, grant number 1.06.03.
DECLARATION OF INTEREST
None.







