Basic Physiology
Normal reproductive function in women involves repetitive cycles of follicle development, ovulation and preparation of the endometrium for implantation should conception occur in that cycle.
Menstruation is a tightly regulated process controlled by the hypothalamic-pituitary-ovarian-endometrial axis.
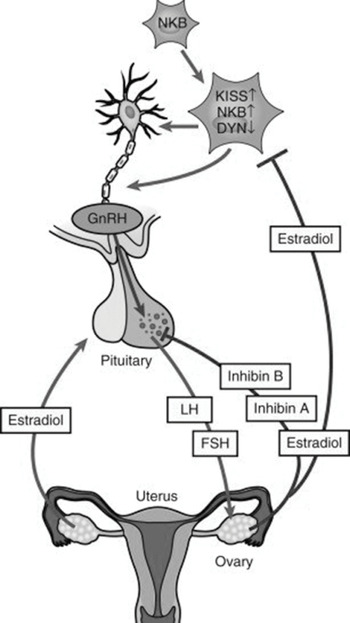
The hypothalamic decapeptide gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile fashion into the pituitary portal venous system.
GnRH regulates the synthesis and subsequent release of follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) from gonadotropes within the anterior pituitary into the circulation.
FSH and LH stimulate ovarian follicular development, ovulation, corpus luteum formation and the coordinated secretion of estradiol, progesterone, inhibin A and inhibin B.
Menstruation occurs 14 days following ovulation if fertilization and subsequent implantation do not occur.
In the absence of pregnancy, the corpus luteum regresses causing a sharp decline in progesterone levels resulting in endometrial breakdown and shedding at menstruation.
In industrialized countries, women may expect to menstruate more than 400 times before menopause.
Normal Menstruation
Menstruation is the shedding of the upper portion of the endometrium (functional layer) in response to withdrawal of progesterone following regression of the corpus luteum in the late secretory phase.
The International Federation of Gynecology and Obstetrics has published normal limits for menstrual parameters:
Neuro-Endocrine Hormones
See Figure 1.1.
The pulsatile GnRH release from hypothalamus is essential for menstruation to occur.
The GnRH pulse generator consists of scattered GnRH neurons within the arcuate nucleus of the medial basal hypothalamus and the preoptic area of the hypothalamus.
Its pulsatile secretion depends on the coordinated action of these scattered neurons.
Kisspeptin, neurokinin B and dynorphin are important regulators of GnRH synthesis and secretion and transduce gonadal feedback signals to GnRH neurons.
The ovarian hormones estradiol and progesterone regulate the output of FSH and LH.
FSH starts to rise a few days prior to the onset of menstruation. It is responsible for recruiting a cohort of ovarian follicles, emergence and growth of a dominant follicle, and for activating follicle aromatase activity, which results in the conversion of androgens to estrogens.
Estradiol has a negative feedback effect on FSH secretion. This is a direct effect of estradiol coupled to its receptor causing repression of FSH transcription.
Steadily rising and sustained high levels of estradiol have a positive feedback effect, leading to enhancement of gonadotropin secretion – an FSH/LH surge.
FSH/LH increases gradually through the follicular phase of the cycle. The high estradiol levels from the dominant follicle cause a mid-cycle LH surge that triggers ovulation.
High concentration of progesterone secreted by the corpus luteum in the secretory phase of the cycle inhibits the secretion of FSH and LH through its actions on both the anterior pituitary gland and the hypothalamus.
The corpus luteum regresses without LH support and luteolysis takes place, converting the corpus luteum to corpus albicans.
The subsequent decline in circulating progesterone results in shedding of the endometrium at menstruation. The negative feedback effects are relaxed and FSH and LH levels start to rise again, initiating another cycle.
If fertilization has taken place, then the corpus luteum is initially maintained by human chorionic gonadotrophin, which is produced by the early embryo.
Menarche
The first menstrual bleed, or menarche, occurs between the ages of 10 and 16 years in most girls in the developed countries.
Control of the GnRH pulse generator is multifactorial and many factors such as ethnicity and genetic heritability have a role to play as well.
The leptin does not appear to be the trigger for reactivation of the GnRH pulse generator but appears essential for the sustained activity of the hypothalamic‐pituitary‐gonadal axis during puberty and adulthood.
Once pulsatile GnRH release is initiated, there is a resulting increase in pulsatile FSH and LH secretion.
The pulsatile GnRH is first at night in low frequency before gradually establishing to the adult pattern of 24‐hour pulsations:
These pulses then stimulate a rise in estradiol levels. The process of puberty takes several years, with menstruation being a late feature.
Ovarian Morphogenesis
Germ cells play an indispensable role in the induction of gonadal development.
Two functional X chromosomes are required for normal ovarian morphogenesis.
Three competing processes – mitosis, meiosis and atresia – determine the temporal pattern of germ cell accumulation and decline in the prenatal ovary.
Ovarian Follicle Development and Ovulation
See Figure 1.2.
The ovary contains approximately one to two million oocytes at birth. The majority become atretic which decreases to about 250,000 oocytes by the time of menarche.
The oocytes are arrested in prophase I of meiosis and held within flattened mesenchymal cells to form primordial follicles. The primordial follicles constitute the ovarian follicular reserve.
The primordial follicle passes through three stages of development before ovulation:
✓ a primary follicle
✓ then a secondary follicle
✓ followed by a pre-ovulatory follicle.
It takes 14 days for a secondary follicle to develop into a pre-ovulatory follicle during the follicular phase of the menstrual cycle.
The timescales for the development are greater than 120 days for the primary follicle to reach the secondary follicle stage, and even longer for the development of primordial follicles to primary follicles.
The expression of oocyte specific genes is essential for normal follicular development.
The oocyte and somatic cells (granulosa and theca cells) of the follicle engage in a complex conversation involving transfer of metabolites through gap junctions, and an autocrine and paracrine dialogue, mainly through members of the transforming growth factor-β family.
The first stage of development to a primary follicle involves protein synthesis and secretion of glycoproteins to form an acellular zona pellucida layer which separates the oocyte from the surrounding granulosa cells. The surrounding stromal cells then condense to form the theca of the follicle, consisting of a vascular inner glandular theca interna and a surrounding fibrous theca externa.
Ongoing progression to secondary follicle involves further proliferation of the granulosa cells and the development of a liquid filled cavity called antrum surrounding the oocyte. This fluid is composed of granulosa cell secretions and serum transudate.
A group of closely associated granulosa cells, called cumulus cells, surround the oocyte. The resulting secondary follicles rely on FSH and LH for their development and without these hormones, atresia of the follicle would occur. See Figure 1.3.
The cyclic recruitment starts after pubertal onset and is the result of the increase in circulating FSH during each reproductive cycle that rescues a cohort of secondary follicles from atresia. Within the secondary follicle, the FSH binds to granulosa cells and theca interna cells interact with LH. The thecal cells synthesize androgens from cholesterol with their production being stimulated by LH.
The granulosa cells have aromatase enzymes which convert androgens to estrogens. Both FSH and estradiol promote granulosa cell proliferation.
There is a surge of estradiol from the most advanced follicle toward the end of the secondary follicle stage. The majority of follicles will undergo atresia and only a subset population will reach the pre-ovulatory stage, with one becoming the dominant follicle.
The selected dominant follicles which have more granulosa cells and higher level of FSH receptors produce more estrogen than subordinate follicles. The granulosa cells produce anti-apoptotic factors upon stimulation by FSH.
The estradiol production in the dominant follicle mediates the effect of FSH and results in the expression of LH receptors on the granulosa cells.
Ovulation is triggered by the pre-ovulatory LH surge, which requires the concerted action of granulosa cell products that regulate expansion of cumulus, production of prostaglandins and progesterone-dependent expression of key enzymes that promote follicular rupture.
Following ovulation, the residual granulosa and theca cells differentiate to form the corpus luteum. The central role of the corpus luteum is the production and secretion of progesterone.
The corpus luteum of the nonfertile cycle undergoes functional and then structural luteolysis, the former being driven by a decline in sensitivity to LH and a fall in steroidogenic acute regulatory protein, followed by apoptosis and autophagy of luteal cells.
Without continued LH secretion, the corpus luteum regresses after 12–14 days in the absence of pregnancy and the withdrawal of progesterone results in menstruation.
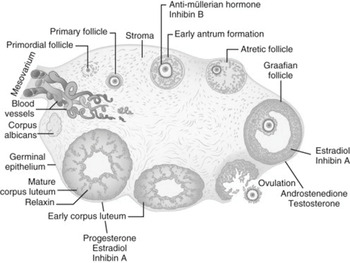
Figure 1.2 The follicular cycle of the human ovary and main secretary products
Endometrium
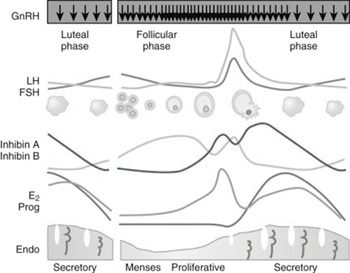
The menstrual cycle is divided into two phases:
✓ proliferative (follicular) phase
✓ secretory (luteal) phase
The endometrium is stratified into two layers:
✓ functional: a superficial transient layer adjacent to the uterine cavity
✓ basal: a deeper permanent layer adjacent to the myometrium.
The functional layer comprises the superficial two‐thirds of the endometrium. It is lined by luminal epithelium and contains glandular epithelium and stroma.
The functional layer of the endometrium is shed during each menstrual cycle and is regenerated during each cycle.
Both estrogen and progesterone influence the development of endometrium at each stage of the menstrual cycle. They act through the estrogen receptor (ER) and progesterone receptor (PR), resulting in initiation of gene transcription and consequent downstream cellular events.
Estrogen is responsible for endometrial proliferation and vascularization, and can also upregulate a number of genes, including PR.
ER expression in the glandular and stromal cells has been found to increase in the proliferative phase before declining in the secretory phase due to the action of progesterone.
PR persists in the stromal compartment of the functional layer during the secretory phase. These cells will retain their progesterone responsiveness and are thus able to respond to progesterone withdrawal, hence leading to onset of menstruation.
Menstruation
Menstruation is a tightly controlled inflammatory process involving the endocrine, immunological and hematological systems. Progesterone withdrawal leads to local release of inflammatory mediators and subsequent influx of leukocytes. This results in release and activation of matrix metalloproteinases (MMP), leading to endometrial tissue breakdown.
A two‐step hypothesis has been proposed with an initial progesterone-dependent step followed by progesterone‐independent events.
In the first progesterone‐dependent step, progesterone withdrawal initiates an inflammatory cascade. Increased inflammatory cytokine and chemokine expression results in an influx of inflammatory cells, which interact with the decidualized stromal cells to mediate the release of pro-inflammatory mediators such as cytokines and prostaglandins.
Progesterone withdrawal leads to up-regulation of endometrial cyclooxygenase-2 and subsequent increased levels of prostaglandins (PGs), namely, PGE2 and PGF2α.
Progesterone withdrawal is also thought to initiate spiral artery vasoconstriction, which causes transient hypoxia in the uppermost endometrium. This hypoxia induces multiple endometrial repair factors, including vascular endothelial growth factor.
The falling circulating progesterone promotes endometrial MMP activity which regulates the degradation of all the components of the extracellular matrix (ECM), causing tissue and vascular destruction and loss of structural integrity, widely accepted as final effectors of menstruation.
In the late secretory phase, there is a significant increase in the endometrial leukocyte population. These leukocytes include T cells, macrophages/dendritic cells, natural killer (NK) cells, neutrophils and mast cells, which produce chemokines and so attract further leukocytes in a positive feedback loop.
Fibrinolysis pathway involves the conversion of the inactive proenzyme plasminogen to plasmin, which then degrades fibrin deposits. Tissue plasminogen activator and urokinase plasminogen activator drive the production of plasmin.
In contrast, plasminogen activator inhibitor inhibits fibrinolytic activity. The falling levels of ovarian steroid hormones in the late secretory phase remove inhibitor of plasmin activity.
The levels of plasminogen activator increase premenstrually. The destruction of the ECM is enhanced, clot organization is prevented and tissue fragments will pass through the cervical os during menstruation.
Control of Bleeding
A number of mechanisms appear to exist in order to limit the menstrual bleeding.
Vasoconstriction and activation of the clotting cascade are more important in achieving hemostasis post-menstruation.
A number of vasoconstrictors such as endothelin‐1 and PGF2α are thought to contribute to this vasoconstriction of the spiral arterioles.
The coagulation cascade is activated by two pathways: extrinsic and intrinsic. Each culminates in the conversion of factor X to Xa, which catalyzes the conversion of pro-thrombin to thrombin, ultimately leading to the formation of a fibrin clot.
Platelet aggregation stimulating the coagulation cascade and formation of a fibrin clot in endometrial vascular repair is relatively low.
Another mechanism of controlling bleeding may involve local endometrial MMP activity.
Endometrial Repair
Tissue formation and remodeling are the key processes in wound repair. Endometrial repair post-menses is usually scar-free and rapidly occurring over three to five days.
Progesterone withdrawal is the trigger for both menstruation and endometrial repair.
Hypoxia likely plays a pivotal role in the endometrial repair process.
Re‐epithelialization of the endometrium, the initial stage of repair, occurs before menstruation ceases and in the absence of ovarian hormones. The rapid repair of the injured blood vessels must also occur to stop endometrial bleeding.
The epithelial cells grow from the necks of the endometrial glands and spread to meet migrating cells from other glands, forming a new luminal surface.
The endometrial shedding and regrowth are piecemeal and occur simultaneously in different areas of the uterus.
A further method of endometrial repair may involve endometrial stem cells.
Proliferative Phase
In the proliferative phase, initial scarless repair is followed by proliferation and angiogenesis in order to regenerate an endometrium which is receptive to possible embryo implantation in the secretory phase following ovulation.
An intact epithelial layer is required before the stromal layer can respond to ovarian hormones.
Estrogens influence endothelial cell growth, angiogenesis and the proliferation to regenerate the endometrium throughout the functional layer from the mid-proliferative phase onwards.
A large number of cytokines and growth factors such as adrenomedullin and connective tissue growth factor may modulate the action of the ovarian steroid hormones.
The human endometrium grows 4–7 mm within four to ten days every menstrual cycle. Despite high estradiol levels, endometrial thickness plateaus at day 9–10 of the menstrual cycle.
Secretory Phase
After ovulation, the progesterone‐dominant secretory phase commences 14 days prior to next menstruation.
Progesterone inhibits epithelial cell mitosis by down-regulating the ER on epithelial cells thus attenuating estrogen’s proliferative effect.
Decidualization is a postovulatory process of endometrial remodeling in preparation for pregnancy, which includes secretory transformation of the uterine glands, influx of specialized uterine natural killer cells (uNK), and vascular remodeling.
The decidualization is a morphological change which is initiated during the mid‐secretary phase of the menstrual cycle as a result of elevated progesterone levels. This leads to stromal cells surrounding the spiral arteries in the upper two‐thirds of the endometrium, regardless of the presence or absence of a conceptus.
The endometrial vasculature consists of the basal arteries, which supply the basal layers, and the spiral arteries which supply the functional layer. Each spiral arteriole originates from a radial artery at the myometrial/endometrial border. It then coils through the endometrium to supply the subepithelial plexus.
The vascular smooth muscle cell proliferation is key to arteriole growth and reported to be increased in the spiral arterioles in the mid to late secretory phase, which enables vessel growth and coiling.
The endometrium contains an immune cell population which responds to changes in endocrine signals across the menstrual cycle.
NK cells are a lymphocyte population with cytotoxic and cytokine‐producing functions.
There is a rise in the number of uNK cells in the late secretory phase of the cycle which may play an important role in spiral arteriole maturation.
There are two populations of NK cells in humans: the cytotoxic NK cells and the cytokine‐producing NK cells.
The uNK cells are a distinct subtype from the peripheral blood NK cells. If pregnancy occurs, the number of uNK cells continues to increase and they are abundant in early gestation. In addition to their role in spiral artery remodeling, they are also thought to play a key role in placentation by controlling trophoblast invasion.
The activity of uNK cells have been shown to be controlled by regional steroid hormones as well as local chemokines, including interleukin (IL)-15.
Decidualization involves changes not only to the spiral arteries and local immune cell population, but also the ECM, glandular epithelium and stromal cells.
The elongated, fibroblast‐like endometrial stromal cells in the proliferative phase become increasingly spherical decidual cells through the action of progesterone, and there is secretory transformation of the glands. Under progesterone exposure, subnuclear glycogen‐rich vacuoles appear in the glandular epithelium and a number of progesterone‐regulated proteins are secreted, including prolactin and insulin‐like growth factor binding protein‐1.
These glandular secretions peak during the midsecretory phase, which coincides with the timing of blastocyst implantation if fertilization has taken place.
If implantation occurs, then decidualization continues into pregnancy. However, in the absence of fertilization, progesterone levels decline due to atresia of the corpus luteum in the late secretory phase.
This decline in progesterone levels initiates a sequence of events resulting in menstruation: subepithelial hemorrhage, the development of linear cracks in the endometrium, and subsequent endometrial shedding.
Ovarian Aging and Insufficiency
Ovarian aging is influenced by genetic variation, hypoxia, environmental factors (including smoking and exposure to environmental toxins) and their interaction with genes controlling follicular complement and quality.
Premature ovarian insufficiency is caused by mutations or variants in genes encoded by the X chromosome as well as autosomes.
Premutations of FMR1 gene on the X chromosome are among the more common variants associated with premature ovarian insufficiency.
Measurements of antimullerian hormone and antral follicle counts have emerged as the most predictive measures of ovarian reserve.
Summary
The human menstrual cycle is complex and tightly controlled by the prevailing endocrine environment.
A better understanding of the mechanisms controlling endometrial function would enable the development of new therapeutics to help manage debilitating menstrual bleeding complaints.