INTRODUCTION
The total dissolved carbon (TDC) content of environmental water samples is presented as a form of dissolved inorganic carbonates (DIC) and in several dissolved organic forms (DOC) (Bisutti et al. Reference Bisutti, Hilke and Raessler2004). The dissolved carbon concentration of these waters varies between a few tens to a few hundred mg L–1. Generally, carbon is mainly bound to the inorganic fraction in the water and only few mg L–1 is bound to the organic compounds. Using the conventional radiometric 14C measurement methods (LSC or GPC), 30–100 L of water sample is needed to obtain accurate analytical results, even for the measurement of the dissolved inorganic (DI14C) fraction (Varlam et al. Reference Varlam, Stefanescu, Varlam, Popescu and Faurescu2007). Due to the above, the determination of the 14C concentration of environmental water samples is generally based on the measurement of the DIC fraction only (Geyh Reference Geyh2000; Gonfiantini et al. Reference Gonfiantini and Zuppi2003).
Radiocarbon (14C) has a decisive importance for the environmental monitoring of nuclear facilities and radioactive waste repositories, as it can spread well with groundwater and indicates the propagation path of the contamination (UNSCEAR 2000). However, to monitor the DIC fraction alone is not sufficient to control the emission, as radioactive wastes may also contain significant amounts of organic compounds with high 14C activity concentration. Moreover, the anthropogenic origin inorganic fraction may be diluted by the relatively high amount of dissolved natural carbonate content of the groundwater, which can reduce the sensitivity of these methods for detection of the environmental impact of the monitored facilities. The dilution and reduced sensitivity phenomenon are less significant in case of the organic compounds due to the much lower natural DOC appearance in groundwater (Wolstenholme et al. Reference Wolstenholme, Cook, Mackenzie, Naysmith, Meadows and McDonald1998; Povinec et al. Reference Povinec, Jull and Burr2001; Cook et al. Reference Cook, MacKenzie, Muir, Mackie and Gulliver2004; Huang et al. Reference Huang, Guo, Wu, Zhang, Chen, Zhang, Qin and Guan2015; Muir et al. Reference Muir, Tierney, Cook, MacKinnon, Howe, Heymans, Hughes and Xu2017).
Previously, several investigations of the organic 14C source mediums were carried out at the Paks Nuclear Power Plant (NPP). Based on previously reported 14C activity concentration values from Paks NPP of samples from the primary coolant and cooling ponds the 14C activity concentration of the inorganic fraction at each reactor block are rather similar: 8–10 Bq L–1 (Isotoptech 2016). Veres et al. (Reference Veres, Hertelendi, Uchrin, Csaba, Barnabás, Ormai, Volent and Futo1995) published that in the ventilation stacks of the Paks NPP, the carbon dioxide fraction represents only 5–6% of the total airborne 14C release, so the airborne organic forms release is 20 times higher. Their preliminary results showed that the 14C is mostly in the form of hydrocarbons and its concentration is about 4 kBq L–1 primary water of Paks NPP (Veres et al. Reference Veres, Hertelendi, Uchrin, Csaba, Barnabás, Ormai, Volent and Futo1995).
Additionally, the analyses of dissolved gases in the water of the spent fuel pool show that in some cases it is not only the inorganic fraction contributes to 14C activity but also other gases in organic and volatile forms. In some cases, it could mean twice the DIC 14C activity. Organic 14C, as one of the most important limiting radionuclide and chemical form for radioactive waste disposal, is also present in low and intermediate level radioactive waste from PWR-type nuclear power plants. Its concentration in the evaporation concentrates of Paks NPP are in the order of between 0.1 kBq L–1 and 1.5 kBq L–1 in dissolved organic fraction and the 14C activity concentrations of inorganic fraction are between 3.5 kBq L–1 and 5.5 kBq L–1 (Isotoptech 2018). Consequently, the previous investigations confirm relevance of the organic 14C measurements in the monitoring of nuclear power plants releases, because probably it can also be emitted into the environment.
Several routine methods are available for the determination of the specific 14C content of the DIC fraction of water samples by AMS measurement method. These methods generally use phosphoric acid to recover the inorganic carbon from a 10–20 mL water sample as a form of CO2. However, in this case the organic compounds remain in the reaction vessel (Gudelis et al. Reference Gudelis, Gvozdait, Kubarevičien, Druteikien, Lukoševičius and Šutas2010; Molnár et al. Reference Molnár, Hajdas, Janovics, Rinyu, Synal, Veres and Wacker2013a, Reference Molnár, Janovics, Major, Orsovszki, Gönczi, Veres, Leonard, Castle, Lange, Wacker, Hajdas and Jull2013b). To measure the organic fraction is much more complicated as about 800–1000 mL sample is necessary, since the concentration of the organic fraction is much smaller than the inorganic one. The inorganic fraction should be removed from the sample, before the oxidation of the organic fraction is performed. After removing the inorganic fraction, the sample can be freeze-dried and then the residue combusted, or the oxidation can be done by wet oxidation method by strong acidic reagents or by UV light (Burr et al. Reference Burr, Thomas, Reines, Jeffrey, Courtney, Jull and Lange2001; Leonard et al. Reference Leonard, Castle, Burr, Lange and Thomas2013; Steier et al. Reference Steier, Fasching, Mair, Lieb, Battin, Priller and Golser2013; Huang et al. Reference Huang, Guo, Wu, Zhang, Chen, Zhang, Qin and Guan2015; Svetlik et al. Reference Svetlik, Fejgl, Povinec, Korínková, Tomášková, Pospíchal, Kurfirt, Striegler and Kaufmanová2017). Due to the complications and difficulties described above it is still very uncommon that the 14C in the DOC fraction is measured as a part of a monitoring program around nuclear facilities.
The aim of the study is to develop an easy-to-use AMS-based measurement method to determine the total dissolved 14C activity concentration (TD14C) of water samples. This can be useful for the environmental monitoring of nuclear facilities and radioactive waste repositories, as there is a lack of data in literature related to the concentration of the 14C found under different chemical forms (Svetlik et al. Reference Svetlik, Fejgl, Povinec, Korínková, Tomášková, Pospíchal, Kurfirt, Striegler and Kaufmanová2017).
EXPERIMENTAL
Instrumentation and Samples
In this study, the specific 14C content of groundwater monitoring in well water (from 12 monitoring wells) collected from the Paks NPP site and the Danube above Paks and the hot water channel were investigated. The activity concentration of inorganic 14C in surface and near-surface groundwater is considered to be nearly constant for the first two factors and is well known. Thus, around nuclear facility, the study of this isotope is an excellent indicator of uncontrolled releases and their detection.
The 14C AMS measurements and sample preparation were carried out in the HEKAL AMS laboratory in Debrecen, Hungary. The introduce of the MICADAS type AMS was detailed in status report in 2013 (Molnár et al. Reference Molnár, Janovics, Major, Orsovszki, Gönczi, Veres, Leonard, Castle, Lange, Wacker, Hajdas and Jull2013b). The concentration of the carbon forms in our samples was measured by Shimadzu® TOC-VCPN analyser according to the EN ISO 1484:1998 standard. This device can measure independently the TDC the DIC and also the DOC concentration of the water. The carbon content details were used for DO14C calculation.
Sample Preparation and Measurement
The organic compounds of environmental water samples can be quantitatively oxidised to CO2 in the presence of the mixture of sulphuric acid, potassium-dichromate and silver-sulphate, a reaction which is used for the determination of the chemical oxygen demand (COD) of water samples (ISO 1989). According to the ISO standard, the reaction is done in an open reaction vessel and the COD is determined by titration. In this way, the released CO2 escapes from the vessel.
In our case, in order to retain the generated CO2, the ISO procedure was modified and the reaction was run in a closed glass vessel specially designed for this purpose (Figure 1). The sample injection port was integrated into the stem of the valve and sealed with PTFE coated septum. This way the reagents can only come in contact with the glass and PTFE parts. The volume of the vessel is 70 mL.

Figure 1 TD14C reaction vessel. W: injection of water sample; PTFE S: PTFE coated septum; V: J. Young high-vacuum PTFE valve; O: oxidizing solution.
The recipe of the ISO standard was further modified in order to add the oxidising agent in a single step. The individual Ag2SO4-H2SO4 and K2Cr2O7-H2SO4 solutions were not prepared separately, but all of the reagents were combined in one solution. The recipe for the modified oxidising solution is the following Eq. (1):
During the sample preparation, 6 mL oxidizing solution is first added into the open reaction vessel by a glass syringe and then evacuated to a 4·10–3 mbar pressure using vacuum line, followed by the sealing of the vessel by the PTFE valve. In the next step, 20 mL water sample is injected through a 0.45 µm syringe filter into the closed vessel through the PTFE coated septum. Then the reaction cell is heated up to 120°C for 2 hr in a heating block to help DOC digestion by the oxidising agents. After the digestion, the cooled reaction vessel is connected to a dedicated gas handling line where the CO2 can be recovered and purified by cryogenic method. The water vapor is trapped first at –70°C (isopropyl alcohol—dry ice) and the CO2 is frozen at –197°C (liquid N2). After the remaining incondensible gases were evacuated, the CO2 was expanded to a known volume and its quantity was measured with Baratron® 626B pressure sensor (range: 0–500 mbar) (Molnár et al. Reference Molnár, Janovics, Major, Orsovszki, Gönczi, Veres, Leonard, Castle, Lange, Wacker, Hajdas and Jull2013b).
The recovered CO2 was converted to graphite by the sealed tube graphitization technique (Rinyu et al. Reference Rinyu, Molnár, Major, Nagy, Veres, Kimák, Wacker and Synal2013). The 14C activities of the graphite targets were measured by our EnvironMICADAS AMS. The overall measurement uncertainty was calculated including counting statistics, background subtraction and normalization (Synal et al. Reference Synal, Stocker and Suter2007). The results were corrected for the δ13C isotopic fractionation using the Bats software (Stuiver and Polach Reference Stuiver and Polach1977; Wacker et al. Reference Wacker, Bonani, Friedrich, Hajdas, Kromer, Němec, Ruff, Suter, Synal and Vockenhuber2010).
RESULTS AND DISCUSSION
Results of the Measurements of Reference Samples
To test for any initial carbon content in the oxidising solution, the COD preparation was executed without the addition of any water sample into the oxidising agent. During these tests significant amount of CO2 was generated (0.006 ± 0.004 mg/6 mL). To get rid of the carbon contamination of the oxidising solution, it was preheated to 120°C for 6 hr in the reaction vessel and the liberated CO2 was purged out by N2 gas flow. We used carbon-free deionized water (MilliQ®, Merck) for making some model solutions during the tests. The possible carbon contribution of the MilliQ water was also tested by the COD method and no measurable CO2 formation was found.
The solution was tested by organic and inorganic reference material, since the TDC contains these two fractions, which are oxidized during digestion. The efficiency of the oxidation was tested by the preparation of 5–5 parallel model samples made of different types of IAEA 14C reference materials (IAEA-C1 to C9) with known carbon content and 14C concentration (Table 1). The model solutions contained 50 mg L–1 of carbon. In case of the insoluble materials, the reference material and the MillQ® water were added to the reaction vessel before the oxidising solution. Independently of the sample type, the carbon content of the model samples was recovered with a yield of 77 ± 2% with good reproducibility. Based on our previous experience, 25–30% of the developed CO2 remained in the liquid phase in the vessel due to the equilibrium solubility of the CO2 (Molnár et al. Reference Molnár, Hajdas, Janovics, Rinyu, Synal, Veres and Wacker2013a). When the CO2 extraction was repeated three times, a yield of 75–80% could be obtained. Considering the results, the conversion factor of the samples to CO2 during the applied oxidation process was close to 100%.
Table 1 Results of the tested IAEA reference materials (no chemical blank correction applied).

1 Gröning et al. (Reference Gröning2007).
According to the above test results, the applied COD preparation method adds only a negligible contamination with modern carbon (∼103 percent modern carbon [pMC]) to the samples, as the results of the fossil (14C free) samples (IAEA-C1 and C9) are very close to their consensus values (close to 0 pMC; Table 1). Contamination of sample preparation for real samples can be taken for correction using the results of the reference (blank) samples. The observed contamination is not higher than 0.01 mg of recent carbon in the case of the applied COD method. For the higher activity IAEA reference material samples (IAEA-C2, C3 and C6), the measured values were in very good agreement with the expected nominal values, within 2 sigma uncertainty level, even without any chemical blank correction. Using the applied COD preparation method, the background is lower than 2 pMC (0.02 fM) for total carbon 14C determination.
Calculating the 14C Activity of the Organic Fraction
With the sample preparation method introduced above, the 14C activity concentration of the total dissolved carbon (TDC) of water samples can be determined as easily as that of the inorganic fraction (DIC). If besides the total dissolved carbon fraction, the 14C activity concentration of the inorganic carbon is also measured and the concentration of all the three carbon fractions is determined, then the specific 14C activity concentration of the dissolved organic carbon (DO14C) can be calculated with the formula below (Eq. 2):
where TD14C, DI14C and DO14C are the specific 14C activities (in pMC units) of the carbon of the total dissolved carbon (TDC), inorganic carbon (DIC) and dissolved organic carbon (DOC, calculated as TDC-DIC) fractions, respectively, wherease TDC, DIC, and DOC are the measured dissolved carbon content concentrations (in mg/L units) for each fraction, respectively.
By considering the propagation of uncertainties, the uncertainty of the calculated specific 14C activity value of the organic fraction (σDO14C) can be expressed as below (Eq. 3):
For the monitoring of the nuclear facilities and for dose calculation, the absolute 14C activity concentration [Bq L–1] of the water is also important which can be expressed using Eq. (4):
where A means the 14C activity concentration of the water sample [Bq L–1]; pMx means the “absolute” percent Modern of the given carbon fraction which is decreasing with time and depends on the date of measurement (which is August of 2014 in this study), Cx means the carbon concentration of the water sample [mgC L–1].
The “absolute” percent Modern of the sample was calculated as Eq. (5):
where, ASN is the normalized specific activity of the sample, and Aabs is the specific activity of the absolute 14C standard (Aabs=226 Bq/kg C) (Mook and van der Plicht Reference Mook and van der Plicht1999; Stenström et al. Reference Stenström, Skog, Georgiadou, Genberg and Johansson2011).
Results of Real, Environmental Samples
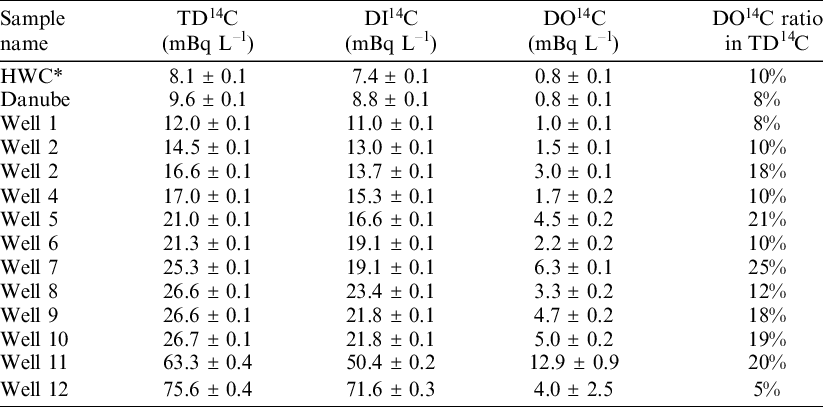
On the site of the Paks NPP, there is a groundwater monitoring network to check the possible radioactive contaminations entering into the groundwater system. Additionally, the site-specific hydrological and contamination dispersion model is also used to identify any possible uncontrolled discharge points. From the groundwater monitoring wells, water samples were taken to measure TD14C, DI14C and the concentration of the different carbon forms. Table 2 shows the AMS 14C measurement results, where the DO14C values are calculated according to the above method. In some cases of certain monitoring wells, significant anthropogenic 14C content was obtained in the dissolved carbon as it was already reported by Janovics et al. (Reference Janovics, Molnár, Futó, Rinyu, Svingor, Veres, Somogyi and Barnabás2010).
Table 2 14C results of water samples from different monitoring wells at Paks NPP. The given DO14C results are calculated value.

* HWC: hot water channel, where the cooling river water returns to the Danube.
If the specific calculated 14C concentration of DOC fraction is plotted in the function of the specific 14C concentration of the DIC, it is clear that the measurement results fit well to the 1:1 line (Figure 2). As it is well visible on Figure 2, on average, DO14C specific activities are slightly higher (8 ± 12%, for the investigated 14 samples) than the DI14C results of the same water sample. Activity concentration of the groundwater samples are highly influenced by the absolute carbon concentrations of targeted fraction. This shows that if we needed a complete picture about 14C distribution in the groundwater system, TD14C and DO14C analyses could be rather useful, besides the commonly applied DI14C measurements.

Figure 2 Specific 14C concentration of the dissolved organic carbon DO14C of the Paks NNP’s monitoring wells in the function of the dissolved inorganic carbon values (the result over 500 pMC of well 12 is not presented on the figure).
Based on these measurements it can be stated that the total carbon content of our water samples is within a relatively narrow range. The mean TDC content of the samples were 79 ± 14 mg L–1. The major part, i.e., 75–95% of TDC was bound to inorganic carbonates, while the organic components were only the 5–25% of the total carbon content.
Using the formula described above, the 14C results can also be expressed as absolute activity concentration of the water samples (Table 3). Because the main part of the carbon is in the inorganic form, the absolute activity concentrations of the TDC and the DIC are close to each other. Regarding the results of our investigations over the 14 different water samples around the Paks NPP (Figure 3), the DO14C contribution to the total 14C activity changes between 5 and 25%.
Table 3 Absolute activity concentrations for the different carbon fractions in the monitoring well of the Paks NPP
* HWC: hot water channel, where the cooling river water returns to the Danube.

Figure 3 Contribution of DI14C and DO14C to the total dissolved carbon 14C activity of the measured groundwater samples.
CONCLUSION
Previous studies have demonstrated the importance of organic 14C measurements in the environmental monitoring of PWR NPPs, as a condition of the technological system, engineering barriers can be controlled and assessed by analyses of organic fraction. One of disadvantages of the only inorganic carbon determination is that the DIC concentration has a significant dilution effect by the natural 14C content of the groundwater on any discharged pollution surrounding the power plant. The total amount of 14C contamination released to groundwater, and hence the exact radiation dose contribution, can be assured with detection and measurement of the total amount of the released (radio)carbon.
Monitoring of uncontrolled 14C emissions of nuclear facilities into groundwater is nowadays performed mainly on the basis of the measurement of the inorganic fraction, nowadays. Within the framework of this study, a simple wet oxidation method was developed for the AMS 14C measurement technique to determine the 14C activity concentration of the total dissolved carbon content of water samples. By the separate measurement of the inorganic and total 14C content in the groundwater in the monitoring wells of the Paks Nuclear Power Plant it was experienced that the 14C contamination getting into the groundwater of the power plant is emitted mostly in inorganic form but can be significant 14C contribution (up to 20%) from the organic fraction which increases the total uncontrolled 14C release. DOC contribution to the TDC 14C activity concentration might be rather higher for the groundwater around radioactive waste storage facilities, especially if significant amount of 14C labelled organic waste is restored in them. During the investigation was obtained between 83.9 and 513.7 pM in TD14C value in monitoring wells of Paks NPP. The calculated DO14C results show that organic fraction can be higher than inorganic fraction. The results of our investigations over the 14 different water samples around the Paks NPP show that DO14C contribution to the total 14C activity concentration was about 5–25% in average.
The elaborated method may be especially useful for environmental monitoring analyses of nuclear facilities and radioactive waste disposal facilities, as with the help of this method, the total 14C activity of groundwater can be determined as easily as the commonly applied DI14C analyses. This way, detection of 14C contaminations becomes possible also in the dissolved organic fraction having been rarely analyzed.
ACKNOWLEDGMENTS
This article was prepared with the professional support of the “Doctoral Student Scholarship Program of the Co-operative Doctoral Program of the Ministry of Innovation and Technology financed from the National Research, Development and Innovation Fund.” The research was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund in the project of GINOP-2.3.4-15-2020-00007 “INTERACT.”











