Introduction
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, we have learned a lot about the development, clinical signs and symptoms, diagnosis, treatment, course and outcome of the disease (Refs Reference Gandhi, Lynch and Del Rio1, Reference Siddiqi and Mehra2). The course of COVID-19 includes multiple stages, which also determines the indicated treatment strategy (Refs Reference Gandhi, Lynch and Del Rio1–Reference Szekanecz3). Stage 1 is the period of early viral infection with fever, respiratory or gastrointestinal symptoms and lymphopenia. Stage 2 is the pulmonary phase. It is divided into two substages: the non-hypoxemic Stage 2a and the hypoxemic Stage 2b. Finally, Stage 3 is the phase of a multisystemic inflammatory syndrome (MIS), occasionally accompanied by the cytokine storm as a pathogenetic feature (Refs Reference Gandhi, Lynch and Del Rio1–Reference Szekanecz3). It is important to note that a real ‘cytokine storm’ occurs in only 2% of patients and in 8–11% of severe patients (Ref. Reference Merrill4). This late stage of COVID-19 also involves, among other mechanisms, bradykinin storm (Ref. Reference Garvin5), the activation of coagulation and complement cascades (Ref. Reference Ackermann6), endotheliitis, vascular leak and oedema (Ref. Reference Ackermann6), microthrombotic events (Ref. Reference Merrill4) and neutrophil extracellular traps (NET) (Ref. Reference Borges7). As these anti-inflammatory agents are most effective during MIS, this should be confirmed by clinical, imaging and laboratory markers (Refs Reference Gandhi, Lynch and Del Rio1, Reference Hu, Huang and Yin8–Reference Caricchio10). Laboratory biomarkers, such as C-reactive protein, ferritin, D-dimer, cardiac troponin (cTn), NT-proBNP, lymphopenia, neutrophil-to-lymphocyte ratio, and, if available, circulating interleukin 6 (IL-6) levels have been associated with MIS in Stages 2b-3 and also with the outcome of COVID-19 (Refs Reference Webb9–Reference Karimi11).
As rheumatologists and immunologists, we encounter a number of important issues with special relevance for autoimmunity, as well as rheumatic and musculoskeletal diseases (RMD). There may be multiple interactions between COVID-19, autoimmunity, systemic inflammation and RMDs. (1) COVID-19 may increase the risk of autoantibody production and autoimmunity (Ref. Reference Ehrenfeld12). (2) Autoimmune-inflammatory RMDs may increase susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Ref. Reference Akiyama13). (3) It is now clear that in the more advanced stages of COVID-19, systemic inflammation and MIS rather than the original viral infection may dominate the clinical picture (Refs Reference Szekanecz3, Reference Hu, Huang and Yin8, Reference Webb9, Reference Mehta14–Reference Constantin16). (4) As a consequence of the above, immunosuppressive drugs successfully used for the treatment of RMDs, such as corticosteroids, biologics or Janus kinase (JAK) inhibitors may also be applied to patients with severe COVID-19 and systemic inflammation (drug repurposing) (Refs Reference Alunno17–Reference Favalli19). (5) It is also crucial how to manage patients with autoimmune RMDs during COVID-19 (Refs Reference Landewe20–23). (6) Vaccination of RMD patients against SARS-CoV-2 is also a fundamental issue (Refs Reference Curtis24, Reference Bijlsma25). Here we will briefly discuss all of these issues associated with autoimmunity, MIS and RMD patients.
SARS-CoV-2 infection, autoimmunity and autoimmune diseases
The development of autoimmunity has been previously described in connection with viral infections other than SARS-CoV-2, such as Epstein-Barr virus (EBV), Cytomegalovirus (CMV), parvovirus B19, hepatitis B (HBV) and C viruses (HCV) (Refs Reference Ehrenfeld12, Reference Fujinami26–Reference Galeotti and Bayry28). Regarding the pathogenesis of autoimmune reactions, at least 30 epitopes (hexapeptides) have been described within both the SARS-CoV-2 spike (S) protein and proteins in human organs allowing the development of autoimmunity through cross-reactivity including molecular mimicry and/or bystander activation. These proteins include ribosomal proteins, methyltransferases, cytokines, such as interleukin 7 (IL-7), as well as lysosomal, sodium channel, cell adhesion, myosin proteins and others (Ref. Reference Ehrenfeld12). This molecular cross-reactivity results in the production of numerous autoantibodies described later, as well as the activation of autoreactive T-cells (Refs Reference Fujinami26, Reference Hussein and Rahal27). In addition, epitope spreading, and the presentation of hidden antigens also emerge as mechanisms (Refs Reference Ehrenfeld12, Reference Fujinami26, Reference Hussein and Rahal27). Autoimmune phenomena based on cross-reactivity have already been described in the previous two major coronavirus epidemics, SARS-CoV-1 and MERS-CoV (Refs Reference Hussein and Rahal27–Reference Ramadan and Shaib29). Peptides found in some proteins of SARS-CoV-2 may also cross-react with peptides of the lung alveolar surfactant protein (Ref. Reference Kanduc and Shoenfeld30). As presented later, such molecular mimicry may also play a role in the development of paediatric inflammatory multisystem syndrome (PIMS) (Refs Reference Constantin16, Reference Onouchi31). In addition, the described hexapeptide cross-reactivities discovered may play a role in the clinical symptoms associated with COVID-19. For example, one of histone lysine methyltransferases may play a role in neurodevelopmental disorders, convulsions and behavioural disorders (Ref. Reference Vallianatos and Iwase32), while IL-7 plays a central role in immune regulation and its absence leads to severe lymphopenia (Ref. Reference Ponchel, Cuthbert and Goeb33). Neuropsychiatric abnormalities and lymphopenia are relatively commonly associated with COVID-19 (Refs Reference Bhaskar15, Reference Galeotti and Bayry28). In paediatric MIS discussed later, at least six protein epitopes of the SARS-CoV-2 virus cross-react with the inositol triphosphate 3 kinase C (ITPKC) Kawasaki antigen, suggesting a role for molecular mimicry in the development of this disease (Ref. Reference Onouchi31).
As for COVID-19, inflammation during the course of the disease, MIS, as well as clinical and radiological phenomena suggest that acute autoimmune and autoinflammatory mechanisms are triggered by viral infection (Refs Reference Caso34, Reference Gagiannis35). SARS-CoV-2-induced lung injury (ARDS) is in many respects similar to the acute exacerbation of interstitial lung disease (ILD) associated with autoimmune diseases (Ref. Reference Gagiannis35). In another study, autoantibodies were detected in 20–50% of pneumonia patients associated with COVID-19 (Ref. Reference Zhou36).
The number of identified autoantibodies associated with SARS-CoV-2 infection is now more than 20. Primarily antinuclear antibody (ANA) and antibodies against elements of the coagulation cascade, such as antiphospholipid (APLA), anti-prothrombin and anti-heparin antibodies have been described. However, anti-citrullinated protein (ACPA), anti-neutrophil cytoplasmic antigen (ANCA), anti-SS-A/Ro antibodies and rheumatoid factor production has also been described with SARS-CoV-2 infection (Refs Reference Ehrenfeld12, Reference Zhou36–Reference Favaloro, Henry and Lippi38).
Some studies have also analysed histological changes induced by SARS-CoV-2 infection. Tissue samples obtained from autopsies of a total of 18 patients who died of COVID-19 showed marked infiltration of T-cells and various T-cell subclasses in the lungs. There have been small cellular infiltrates in the kidney, liver, intestinal wall and pericardium. The dominant cell type was the CD8 + T lymphocyte (Ref. Reference Zinserling39).
Regarding clinical autoimmune syndromes, mainly involvement of the nervous system, such as Guillain-Barré and Miller-Fisher syndrome, as well as immune thrombocytopenia (ITP) have been described, but systemic lupus erythematosus (SLE) or Kawasaki disease (KD) may also occur (Ref. Reference Ehrenfeld12). Thromboembolic complications including deep vein thrombosis of the lower extremity, pulmonary embolism or stroke have been reported in association with severe COVID-19 (Ref. Reference Merrill4). These events may be associated with the autoantibodies described above. For example, while the majority of thromboembolic events occurred in older patients, stroke was also observed in younger patients of 30–50 years of age. The latter may be explained by APLAs (Ref. Reference Zhang40). Indeed, APLAs have been detected in patients with severe thromboembolic COVID-19 (Refs Reference Zhang40, Reference Xiao41). In one study, lupus anticoagulant positivity was found in 45% of 56 COVID-19 patients (Ref. Reference Harzallah, Debliquis and Drenou42), compared with 85% in those admitted to the intensive care unit (ICU) due to ARDS (Ref. Reference Helms43). Lupus anticoagulant correlated with thrombotic events and D-dimer levels (Ref. Reference Helms43). The predominant APLA isotype was IgA (Ref. Reference Zhang40). We have detected IgA APLA in patients with the acute coronary syndrome (Ref. Reference Veres44). Thus, SARS-CoV-2 infection is likely to lead to an IgA-type immune response leading to mucosal damage (Refs Reference Ehrenfeld12, Reference Zhou36). Based on these results, the SARS-CoV-2 virus can induce APLA, which has also been described for other viruses, such as EBV, CMV and HCV (Ref. Reference Ehrenfeld12). It should be noted that APLA production does not always lead to thrombotic events or miscarriages and the occurrence of classical antiphospholipid syndrome (APS) after SARS-CoV-2 infection is rare (Ref. Reference Ehrenfeld12). Therefore, the pathogenetic role of APLA in these events remains to be elucidated (Refs Reference Ehrenfeld12, Reference Shoenfeld45). Fibromyalgia symptoms may also worsen during COVID-19 (Ref. Reference Salaffi46).
The risk and outcome of SARS-CoV-2 infection in adult and paediatric RMD patients
Autoimmune RMDs with high inflammatory activity may increase susceptibility to SARS-CoV-2 infection and worsen the outcome of COVID-19 (Ref. Reference Akiyama13). According to the COVID-19 Global Rheumatology Alliance (C19-GRA) registry, age, previous corticosteroid use and comorbidities of RMD patients are considered to be poor prognostic factors for the development and course of COVID-19. In turn, tumour necrosis factor α (TNF-α) inhibitor biologics, especially in monotherapy, have been associated with better COVID-19 outcomes (Ref. Reference Landewe20).
A recent meta-analysis determined the risk of SARS-CoV-2 infection in RMD patients and the factors influencing this risk. A total of 62 publications were suitable for assessing the prevalence, while 65 papers discussed the outcome of COVID-19 in RMDs. The survey included patients with rheumatoid arthritis (RA), SLE, systemic sclerosis (SSc), spondylarthritis (SpA), Sjögren's syndrome (SS), idiopathic inflammatory myopathies (IIM) and systemic vasculitis. Based on the results of 62 observational studies, a total of 878 cases of COVID-19 were found in 319 025 patients. Thus, in general, the prevalence of COVID-19 was 0.011 [95% confidence interval (CI): 0.005–0.025]. The highest prevalence was observed in SLE, SSc and SS, where long-term corticosteroid use may also have played a role. Based on seven case-control studies, the risk of COVID-19 in RMD patients was 2.19-fold (95% CI: 1.05–4.58) higher than in the general population (P = 0.038). Based on regression analysis, long-term corticosteroid use was an independent factor for the development of COVID-19. In this study, age, gender, hypertension, diabetes mellitus or the use of conventional synthetic (csDMARD), biologic (bDMARD) and targeted synthetic disease-modifying drugs (tsDMARD) were not associated with the risk of COVID-19 (Ref. Reference Akiyama13).
The same group also investigated the clinical outcome of COVID-19 and its determinants. Data from 2766 patients with COVID-19 were reviewed in a total of 65 observational studies. The mean hospitalisation rate was 0.35 (95% CI: 0.23–0.50). The highest demand of hospitalisation was observed in RA and SpA. The COVID-19 mortality in RMD patients was 0.066 (95% CI: 0.036–0.120). Again, mortality in RA and SpA was higher compared to non-RMD patients or healthy individuals in part due to age and comorbidities. Age over 63 years, male gender, hypertension, diabetes mellitus and obesity were associated with a need for hospitalisation, intensive care and mortality. Regarding medication, previous long-term corticosteroid treatment, csDMARD use, as well as b/tsDMARD + csDMARD combination therapy, especially rituximab, negatively affected clinical outcome compared to b/tsDMARD monotherapy. In particular, there was a reduction in hospitalisation and mortality using anti-TNF monotherapy (Ref. Reference Akiyama13). Some reports also suggest that sulfasalazine, abatacept and JAK inhibitors might also worsen COVID-19 outcomes, however, this needs to be confirmed in larger cohorts (Refs Reference Strangfeld47, Reference Sparks48).
In a retrospective study carried out in Spain, patients with chronic inflammatory diseases had a 1.3-fold higher prevalence of hospital COVID-19 compared to the reference population. Patients receiving tsDMARDs or bDMARDs but not those on csDMARDs had a greater prevalence (Ref. Reference Pablos49). In the same cohort, severe COVID-19 was associated with autoimmune RMDs but not with inflammatory arthritis or immunosuppressive therapies (Ref. Reference Pablos50).
At the beginning of the COVID-19 pandemic, it seemed that the disease rarely affected children and if so, the course of the disease was still generally mild. Later, with the spread of new variants, paediatric COVID-19 became more common, and some severe cases were reported. COVID-19-associated paediatric MIS, in many ways similar to the classical KD, has been described in 6–9-year-old children. Clinical symptoms include fever, abdominal pain, mucosal signs, rash, lymph node swelling, hepatosplenomegaly, neurological symptoms and oedema of the hands and feet (Refs Reference Constantin16, Reference Verdoni51). This condition was first termed hyperinflammation syndrome (HIS) but later the term PIMS was introduced and is still used today (Refs Reference Webb9, Reference Constantin16, Reference Verdoni51). The disease is also characterised by high CRP, ferritin and D-dimer levels. In rare, severe cases, it may also meet the criteria for macrophage activation syndrome (MAS) with pancytopenia and extremely high ferritin (Refs Reference Constantin16, Reference Verdoni51). PIMS is similar to KD but also differs in many respects: it affects a wider age group, neurological, cardiovascular and gastrointestinal symptoms are more common, while CRP, platelet and lymphocyte counts are lower than in KD (Refs Reference Constantin16, Reference Verdoni51). KD is more prevalent in infants, while PIMS is more common in 9–14-year-olds (Refs Reference Constantin16, Reference Verdoni51). Cases of similar KD-like diseases have been reported in adults (Ref. Reference Sokolovsky52). Seropositivity for IgG-type anti-SARS-CoV-2 is not detected in the majority of patients (Ref. Reference Constantin16).
Based on all this, the overall risk of SARS-CoV-2 infection in patients with autoimmune RMDs may be higher. The underlying RMD itself is unlikely to result in a higher need for hospitalisation and mortality. However, the outcome of COVID-19 in RMD patients may be associated with prior drug therapy (Ref. Reference Akiyama13). It is of note that the possible harmful effects of previous long-term corticosteroid treatment on the outcome of COVID-19 do not contradict the indication of glucocorticoids in COVID-19-associated MIS (Refs Reference Hu, Huang and Yin8, Reference Alunno17).
Repurposing of antirheumatic drugs for the treatment of severe COVID-19
We discussed the different stages of COVID-19 above (Refs Reference Gandhi, Lynch and Del Rio1–Reference Szekanecz3). Patients in Stage 1 with early viraemia and mild symptoms should be treated with antiviral drugs, both small molecules or therapeutic monoclonal antibodies. We will not discuss these agents in the present review. Antirheumatic and anti-inflammatory drugs used in the treatment of RMDs may include corticosteroids, bDMARD and tsDMARDs. These compounds are also used in Stages 2b and 3 of COVID-19 (Refs Reference Szekanecz3, Reference Mehta14, Reference Alunno17).
Antirheumatic drugs currently used to treat COVID-19 are listed in Table 1 and the use of different treatments in various stages of COVID-19 is shown in Figure 1. Of note, relatively few high-value, hard endpoint (mortality, hospitalisation), randomised, controlled trials (RCT) have been performed. In most cases, uncontrolled or minor studies are available, with a few exceptions (e.g., the RECOVERY trials). However, emergency use authorisation (EUA) has been donated to some of these agents due to urgency. First, some information on these anti-inflammatory agents will be discussed followed by the latest recommendations of the European Alliance of Associations for Rheumatology (EULAR).

Fig. 1. The use of anti-COVID-19 therapies at different stages of the disease. Explanations: 1: in stage 3, remdesivir may be used in combination with baricitinib; 2: reconvalescent plasma therapy may be used in immunosuppressed states, as well as in sustained viraemia; 3: corticosteroids might be used in hypoxia and/or MIS; 4Targeted therapies should be applied in MIS. See Table 1 for more details.
Table 1. Antiinflammatory and immunosuppressive agents repurposed for the treatment of COVID-19 (Ref. Reference Szekanecz3)
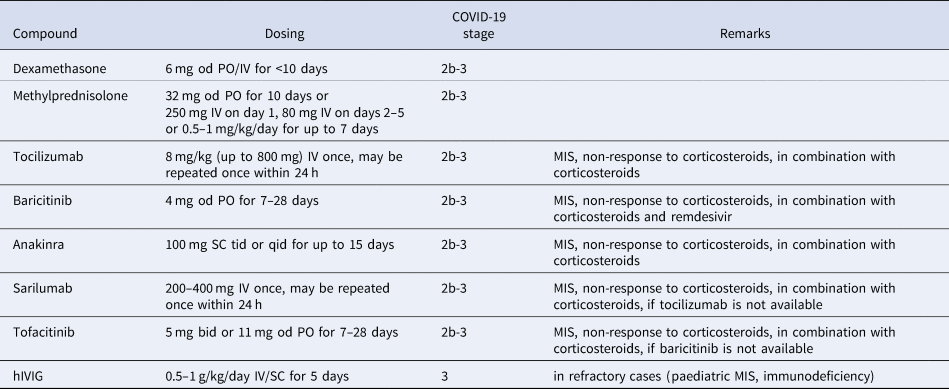
bid, twice daily; hIVIG, human high-dose intravenous immunoglobulin; IV, intravenous; MIS, multisystemic inflammatory syndrome; od, once daily; PO, per os; qid; four times daily; SC, subcutaneously; tid, three times daily.
Corticosteroids, as discussed above, are not recommended in the early stages of SARS-CoV-2 infection as, in retrospective studies in autoimmune RMD patients, previous long-term corticosteroid treatment was associated with increased susceptibility to COVID-19 and disease severity (Ref. Reference Akiyama13). However, in more severe cases of COVID-19 with MIS and organ damage (Stages 2b-3), dexamethasone significantly reduced 28-day mortality. Efficacy was better in those in need for invasive ventilation in the ICU (Ref. Reference Group53). Hospital mortality and clinical outcome were also improved by methylprednisolone in a study conducted by the EULAR working group, particularly in those with marked elevations in CRP, D-dimer and ferritin (Ref. Reference Ramiro54). Dexamethasone has received FDA EUA approval (Ref. 55) (Table 1, Fig. 1).
The IL-6 receptor (IL-6R) inhibitor tocilizumab has been effective in several RCTs in patients in Stages 2b-3 (Refs Reference Xu56–58). In these studies, tocilizumab was effective in Stages 2b-3 in severe cases requiring ICU admission and ventilation. In these patients, tocilizumab improved survival and the chance of hospital discharge (Refs Reference Xu56–58). In the REMAP-CAP study, tocilizumab was so effective within two days of ICU referral that the study was prematurely terminated (Ref. Reference Gordon57). In the CHIC study conducted by EULAR, tocilizumab in combination with corticosteroid improved the clinical picture and survival in patients initially treated with corticosteroids only but who did not show adequate response (Ref. Reference Ramiro54). Finally, the largest COVID-19 therapeutic study to date (RECOVERY; 4116 patients) included patients requiring invasive ventilation, non-invasive oxygen therapy or none of these (control group). At baseline, 82% of the patients received corticosteroids. In hypoxic, Stage 2b-3 patients requiring hospitalisation, tocilizumab in comparison to standard care significantly reduced invasive mechanical ventilation or mortality (35 vs 42%) and improved the chance of hospital discharge within 28 days (57 vs 50%). Tocilizumab also decreased the need for invasive ventilation (Ref. 58) (Table 1, Fig. 1).
On the other hand, tocilizumab was not effective in patients with mild-to-moderate COVID-19 not requiring ICU admission (Refs Reference Stone59, Reference Hermine60). Thus, tocilizumab is recommended in patients with MIS who do not respond to corticosteroids (Refs Reference Ramiro54, Reference Gordon57, 58) but not in the early stages of COVID-19 nor in the absence of significant inflammation (Refs Reference Stone59, Reference Hermine60). It should also be noted that tocilizumab transiently increases circulating IL-6 levels (due to the competitive binding to the IL-6 receptor), therefore, determination of serum IL-6 concentration is only recommended at baseline, before the initiation of treatment (Ref. Reference Sciascia61).
Another IL-6 receptor inhibitor, sarilumab, also improved the survival of patients with severe COVID-19 (REMAP-CAP trial). Sarilumab also increased the probability of ICU and hospital discharge (Ref. Reference Gordon57). In contrast, in a smaller study (SARI-RAF), no clinical improvement or longer survival was observed in patients with pneumonia who did not require invasive ventilation (Ref. Reference Della-Torre62) (Table 1, Fig. 1).
Among the JAK inhibitors, baricitinib has antiviral effects by blocking viral entry through ACE2 into the cell (Ref. Reference Bronte63). Baricitinib also exerts anti-inflammatory effects leading to the reduction of MIS and restoration of immune regulation (Refs Reference Bronte63, Reference Tsai and Tsai64). Baricitinib in combination with remdesivir significantly improved clinical status and reduced time to recovery in severe COVID-19 patients requiring respiratory therapy (Stages 2b-3). On the other hand, baricitinib was not effective in mild to moderate SARS-CoV-2 infection (Ref. Reference Kalil65). FDA approved the use of the baricitinib-remdesivir combination in severe COVID-19 (Ref. 66) (Table 1, Fig. 1).
Another JAK inhibitor, tofacitinib was also effective in the STOP-COVID trial. Hospitalised adults with COVID-19 pneumonia received either tofacitinib 10 mg bid or placebo bid for up to 14 days or until hospital discharge. In this patient population, tofacitinib treatment led to a lower risk of death or respiratory failure through day 28 in comparison to placebo (Ref. Reference Guimaraes67) (Table 1, Fig. 1).
The role of autoinflammation including NLRP3 inflammasome activation, as well as IL-1β and IL-18 production has also been demonstrated in COVID-19. The IL-1 receptor antagonist (IL-1Ra) anakinra improved survival in patients with Stage 2b-3 COVID-19 (Refs Reference Langer-Gould68–Reference Kyriazopoulou70). In contrast, anakinra was not effective in patients with hypoxia not requiring ventilation (Ref. 71). Early increase of plasma soluble urokinase activator receptor (sUPAR) levels has been associated with increased risk of COVID-19 progression. Interestingly, anakinra was particularly effective in patients with high plasma sUPAR (Ref. Reference Kyriazopoulou72). The use of anakinra is also relevant in PIMS (Refs Reference Constantin16, Reference Phadke73) (Table 1, Fig. 1).
There have been very few studies on the use of high-dose human intravenous immunoglobulin (IVIG) in COVID-19. In a controlled study of 59 patients, IVIG resulted in clinical improvement after initial treatment failure (Ref. Reference Gharebaghi74). However, in a similarly designed RCT, IVIG in combination with other antiviral agents was ineffective (Ref. Reference Tabarsi75). In the recent ICAR RCT, IVIG was tried in patients with COVID-19 who received invasive mechanical ventilation for moderate-to-severe ARDS. In these patients, IVIG did not improve clinical outcomes at day 28 (Ref. Reference Mazeraud76). The effect of IVIG on earlier disease stages of COVID-19 is currently being assessed (Ref. Reference Mazeraud77). IVIG may also be administered to children with KD (Ref. Reference Constantin16), as well as to RMD patients with immunodeficiency (Ref. Reference Szekanecz3).
Several other compounds used for the treatment of RMDs have been tested in mostly small COVID-19 studies. In summary, antimalarials including chloroquine and hydroxychloroquine, as well as colchicine, leflunomide or cyclosporine A are not recommended due to controversial study results, a lack of real evidence or side effects (Refs Reference Szekanecz3, Reference Alunno17, Reference Horby78).
Management of patients with autoimmune RMDs during the pandemic
With respect to autoimmune RMD patients, the main issue is their management including the use of immunosuppressive agents during the COVID-19 pandemic. As discussed above, these patients are at high risk of developing COVID-19 (Refs Reference Ehrenfeld12, Reference Akiyama13). The management of already SARS-CoV-2-infected and not infected RMD patients are highly different (Refs Reference Landewe20, Reference Mikuls79).
Regarding immunosuppressive treatment of autoimmune RMD patients, previous long-term corticosteroid therapy was associated with a more severe outcome of COVID-19, while anti-TNF bDMARDs tended to improve it (Ref. Reference Akiyama13). We have less data on non-TNF bDMARDs and tsDMARDs (Refs Reference Landewe20, Reference Mikuls79). In general, the activity of the underlying disease may confer more risk for SARS-CoV-2 infection than csDMARDs, bDMARDs or tsDMARDs (Refs Reference Ehrenfeld12, Reference Favalli19, Reference Landewe20, Reference Mikuls79, Reference Ferro80). In RA, increased inflammatory activity increases the risk of severe infections and the infection itself may cause an exacerbation of the underlying disease (Refs Reference Favalli19, Reference Ferro80, Reference Listing, Gerhold and Zink81). Thereafter, disease activity and infection can create a vicious cycle (Ref. Reference Favalli19).
Autoimmune RMDs may flare up without treatment or after reducing the dose leading to an increased risk of infection (Refs Reference Favalli19, Reference Ferro80, Reference Listing, Gerhold and Zink81). Therefore, it is not recommended to discontinue immunosuppressive therapy, reduce its dose or increase treatment intervals. In an uninfected, stable RMD patient, the continuation of non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, csDMARDs, bDMARDs and tsDMARDs in effective doses is recommended (Refs Reference Favalli19, Reference Landewe20, Reference Mikuls79, Reference Ferro80). ACR also recommends that low-dose corticosteroids (≤10 mg prednisolone equivalent) should be initiated if necessary and continued at the lowest possible dose. In severe, life-threatening RMD, such as lupus nephritis or active systemic vasculitis, high-dose corticosteroid therapy is recommended (Ref. Reference Mikuls79). Abrupt discontinuation of corticosteroids is not recommended even in the setting of severe infection (Refs Reference Favalli19, Reference Mikuls79). There is also no contraindication to re-initiate targeted therapy if justified by disease activity (Refs Reference Favalli19, Reference Landewe20, Reference Mikuls79, Reference Ferro80). Only febrile, acute COVID-19 may be an indication for discontinuation of csDMARDs, bDMARDs and tsDMARDs (Refs Reference Favalli19, Reference Landewe20, Reference Mikuls79, Reference Ferro80). As discussed above, some bDMARD and tsDMARD agents have been introduced to the therapy of Stage 2b-3 COVID-19 with MIS through repurposing (Refs Reference Mehta14, Reference Alunno17). However, it still needs individual decision to continue tocilizumab or tsDMARDs in SARS-CoV-2-infected RMD patients (Refs Reference Landewe20, Reference Mikuls79).
With respect of SARS-CoV-2-infected autoimmune RMD patients, according to the latest ACR recommendations, NSAIDs and sulfasalazine can be continued. DMARDs, with the exception of IL-6 inhibitors, should be temporarily suspended and restarted after 2 weeks of asymptomatic observation. In individual cases, anti-IL-6 receptor therapy may be used in agreement with the patient. In documented COVID-19 disease, regardless of disease severity, all DMARDs should be temporarily discontinued. However, suspension of corticosteroids is not recommended. In mild cases of COVID-19 with no or mild pneumonia requiring outpatient treatment and home quarantine, immunosuppressive agents may be restarted within 7–14 days of asymptomatic disease. Continuation of treatment after severe COVID-19 should be considered on an individual basis (Ref. Reference Mikuls79).
EULAR has initiated a COVID-19 registry and issues a new report regularly (Ref. 82). As of October 2021, the database includes more than 10 300 patients. Two-third of patients are women, with a median age of 55 (Refs Reference Helms43–66) years. Altogether 4% of patients are under 18 years of age and 36% are over 60 years of age. The most common diagnoses are RA (38%), axial or peripheral SpA (16%), PsA (13%) and SLE (6%). About 27% of patients required hospital care. Eighty-one % of patients received DMARDs, of which 45% received targeted therapies (Ref. 82).
Finally, In the Spanish COVIDSER study that include 7782 RMD patients, the use of TNF inhibitors was associated with decreased, while that of rituximab was associated with increased risk of hospitalisation. Yet, in this study, the use of bDMARDs was not associated with COVID-19 severity (Ref. Reference Alvaro Gracia83).
Anti-SARS-CoV-2 vaccination of autoimmune RMD patients
EULAR recommends that all autoimmune RMD patients should be vaccinated against COVID-19. The same is true for patients taking corticosteroids, MTX, bDMARDs or tsDMARDs. For B-cell depleting agents, such as rituximab, vaccination should be performed at least 3 months after the administration of this bDMARD (Ref. Reference Bijlsma25). ACR emphasises that the rheumatologist should monitor the patient's vaccination status and discuss the details of the vaccination. Factors related to the underlying disease, treatment, age, gender should be considered as they may affect the success of vaccination. In principle, patients with autoimmune RMDs should be prioritised for vaccination over the general population because of their increased risk of infection. Exacerbation of the underlying RMD after vaccination is extremely rare and the benefits of vaccination far outweigh the risks. Regarding vaccination practice, ACR points out that none of the FDA-approved vaccines is preferred; the first and second doses of the same vaccine should be administered unless contraindicated; routine laboratory testing, including anti-SARS-CoV-2 antibody testing, before and after vaccination is not recommended; epidemiological regulations must be followed even after the full vaccination course; vaccination of people living together with the RMD patient is also recommended in order to protect the patient; and vaccination should be conducted as soon as possible, regardless of the severity of the underlying RMD, except in the severe conditions when the patient is in the ICU (Ref. Reference Curtis24).
In a recent multicentre observational study, the immunogenicity of the BNT162b2 mRNA vaccine including seropositivity rates and serum anti-S protein titres has been evaluated in autoimmune RMD patients. RA, axSpA, PsA, SLE and large-vessel vasculitis patients exerted seropositivity rates between 82.1 and 96.9% compared to controls (100%). On the other hand, patients with IIM and ANCA-associated vasculitis (AAV) had seropositivity rates of 36.8 and 30.8%, respectively. Similarly, patients excluding IIM and AAV had serum anti-S titres of 108.7–173.1 AU/ml compared to controls (218.6 AU/ml). Anti-S titres of IIM and AAV patients were only 42.9 and 40.3 AU/ml, respectively (Ref. Reference Furer84).
Regarding vaccination of RMD patients taking immunosuppressive drugs, in the multicentre trial described above, corticosteroids, rituximab, abatacept belimumab and mycophenolate mofetil (MMF) in monotherapy or in combination exerted significantly lower seropositivity rates (36–77%) compared to controls. On the other hand, seropositivity rates were acceptable (84–100%) in RMD patients receiving MTX, hydroxychloroquine (HCQ), leflunomide, as well as TNF-α, IL-6, IL-17 and JAK inhibitors (Ref. Reference Furer84). In this regard, there is some inconsistency between ACR (Ref. Reference Curtis24) and EULAR recommendations (Ref. Reference Bijlsma25). ACR does not suggest any changes in the doses or timing of corticosteroids, antimalarials, sulfasalazine, leflunomide, MMF, azathioprine, oral cyclophosphamide (CYC) and bDMARDs. However, by referring to some previous studies, ACR suggests a 2–4-week break in MTX and tsDMARDs after vaccination. ACR also suggests a break after vaccination for abatacept (1 week), intravenous CYC (1 week) and rituximab (2–4 weeks) (Ref. Reference Curtis24). In contrast, EULAR recommends transient suspension for rituximab only (Ref. Reference Bijlsma25). As far as MTX is concerned, it has indeed been suggested in the past in relation to other vaccines (e.g. influenza) that vaccination may be more effective if MTX administration is suspended (Ref. Reference Park85). However, a more recent study has suggested that even a single dose of mRNA vaccine against SARS-CoV-2 is effective without temporary suspension of MTX therapy (Ref. Reference Boyarsky86). In the latter study, antibody responses to the mRNA vaccine were also acceptable in RMD patients receiving other bDMARDs and tsDMARDs with the exception of rituximab (Ref. Reference Boyarsky86). In addition, neither MTX nor bDMARDs affected the humoral and cellular immune responses to mRNA vaccines (Refs Reference Mahil87–Reference Connolly and Paik89). In a recent study using questionnaires, up to 28% of immunosuppressive medications had to be modified around the time of anti-SARS-CoV-2 vaccination. More modifications had to be carried out after the second compared to the first dose. A number of drug modifications were not consistent with recommendations (Ref. Reference Barbhaiya90).
In conclusion, COVID-19 and autoimmune RMDs may have several links. Patients with autoimmune RMD might have increased susceptibility to SARS-CoV-2 infection. The SARS-CoV-2 virus might trigger autoimmunity. Immunosuppressive agents have been introduced to the treatment of MIS associated with COVID-19. Finally, patient care and vaccination of autoimmune RMD patients need special attention during the pandemic.






