Zn is a trace element essential for life. Zn deficiency impairs growth, immune activity and reproductive function; however, Zn can also be toxic if over-accumulated( Reference Yu, Wu and Zhang 1 ). The Zn stores in avians are easily depleted, and thus Zn is commonly supplemented in diets for poultry. The use of organic trace mineral supplements in the feed industry has increased during the last 20 years. However, the results on whether the absorption of organic Zn is more effective than that of inorganic Zn in rats, pigs, dogs and humans are inconsistent( Reference Hill, Peo and Lewis 2 – Reference Beutler, Pankewycz and Brautigan 5 ). Our previous data have demonstrated that organic Zn absorption in three small intestinal segments of broilers was more effective than that of inorganic Zn, and increased with increasing chelation strength (defined as the quotient of formation (Q f), which is a quantitative measurement of complex or chelation strength between metal and ligand)( Reference Holwerda, Albin and Madsen 6 , Reference Yu, Lu and Luo 7 ). In addition, organic Zn, especially that with strong chelation strength, could lessen the negative effect of phytate on Zn absorption in ligated small intestinal segments, and showed higher Zn absorption under a high level of phytate( Reference Yu, Lu and Wang 8 ). However, the findings from this half-in vivo study need to be verified by in vivo studies of broilers. To our knowledge, no in vivo study has been reported on the influence of organic and inorganic Zn sources on Zn absorption in the gut of animals.
An earlier study performed with broilers in our laboratory showed that the kinetics of inorganic Zn absorption followed a saturable carrier-mediated process in either the duodenum or the jejunum, but a non-saturable diffusion process in the ileum, depending on the regulation of Zn transporter expression( Reference Yu, Lu and Luo 7 ). However, whether the absorption mechanism of organic Zn differs from that of inorganic Zn in poultry remains largely unknown. In addition, metallothionein (MT) is believed to function in the transport and sequestration of Zn by intracellular vesicles( Reference Palmiter, Cole and Findley 9 , Reference Davis, McMahon and Cousins 10 ), and we previously found that it participated in regulating inorganic Zn absorption( Reference Yu, Lu and Luo 7 ). However, whether MT is involved in organic Zn absorption has not been elucidated with a kinetic absorption study before, and thus it needs to be further explored using the ligated duodenal loops of broilers, as MT has been shown to be mainly expressed in the duodenum of broilers( Reference Palmiter, Cole and Findley 9 , Reference Davis, McMahon and Cousins 10 ).
Therefore, the present study aimed to determine the effect of organic and inorganic Zn sources on Zn absorption using intact living broilers and to investigate the kinetics of organic and inorganic Zn absorption in the ligated duodenal loops of broilers to elucidate the potential mechanisms of Zn absorption by the broiler intestine.
Methods
Experimental design and treatments
In the present study, a completely randomised design was adopted. Expt 1 was carried out to evaluate the effect of four Zn sources on Zn absorption by the intestine of intact living broilers. A total of five dietary treatment groups were designed, including the control with no Zn supplementation, ZnSO4.7H2O, Zn-amino acid chelate with a weak chelation strength (Zn-AA W, Q f=6·48, 11·93 % Zn by analysis on an as-fed basis), Zn-protein chelate with a moderate chelation strength (Zn-Pro M, Q f=30·73, 13·27 % Zn by analysis on an as-fed basis) and Zn-protein chelate with a strong chelation strength (Zn-Pro S, Q f=944·02, 18·61 % Zn by analysis on an as-fed basis), which were the same as those used in our previous studies( Reference Yu, Lu and Wang 8 , Reference Liu, Lu and Wang 11 ). These organic Zn sources were obtained from independent distributors rather than directly from the product manufacturers.
Expt 2 was conducted to elucidate the possible mechanisms of organic Zn absorption in the intestine of broilers through a study of the kinetics of Zn absorption in the in situ ligated duodenal loops. The duodenal segment was selected because the differences in organic Zn absorption in this section were very obvious as seen in our previous report( Reference Yu, Lu and Wang 8 ). There were a total of twenty-five treatments of Zn injections in a 1 (control) +4 (Zn sources)×6 (injected Zn concentrations) factorial arrangement of treatments. Four Zn sources were the same as those in Expt 1, and injection solutions contained 0 (control), 0·077, 0·154, 0·308, 0·616, 1·232 or 2·464 mmol of Zn/l as one of four Zn sources in perfusates.
Animals and diets
All experimental procedures were approved by the Animal Care and Use Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences. A total of 400 1-d-old birds (Arbor Acres commercial male broilers) were weighed and allotted randomly to one of five treatments with eight replicate cages of ten chicks each in Expt 1, or a total of 250 1-d-old birds were weighed and allotted randomly to one of twenty-five treatments with ten replicate cages of one chick each in Expt 2. The average body weights of birds used for each treatment were nearly equal in order to avoid the effect of weight on the results. The birds were handled in accordance with guidelines approved by the Office of the Beijing Veterinarian. The birds in Expt 1 were fed a Zn-unsupplemented maize–soyabean meal basal diet (27·62 mg of Zn/kg of diet by analysis on an as-fed basis, Table 1) from days 1 to 14 to deplete the body Zn stores, and then fed a Zn-unsupplemented maize–soyabean meal basal diet (28·82 mg of Zn/kg of diet by analysis on an as-fed basis, Table 1) and the basal diet supplemented with 60 mg of Zn/kg of diet according to our previous results( Reference Huang, Lu and Luo 12 ) from one of the above four different Zn sources from days 15 to 28. The birds were fasted overnight on day 14, and then weighed early on day 15 to equalise the body weights in all groups. In order to show the effect of additives on the results of experimental rearing, the birds were weighed on either day 21 or 28 after overnight fasting. Each Zn source was premixed with maize starch to the same weight, and then was added to the respective experimental diet on the basis of its analysed Zn concentration. The birds in Expt 2 were fed a maize–soyabean meal basal diet supplemented with 60 mg of Zn/kg of diet from ZnSO4 (96·42 mg of Zn/kg of diet by analysis on an as-fed basis, Table 2) from days 1 to 21, and then fed a Zn-unsupplemented semi-purified diet (14·61 mg of Zn/kg of diet by analysis on an as-fed basis, Table 2) from days 22 to 28 to deplete the body Zn stores. Variable small amounts of l-lysine monohydrochloride or dl-methionine were added to the respective experimental diets according to the amounts of lysine and methionine from supplemental organic Zn sources so as to balance lysine and methionine in each experimental diet. All other nutrients in the diets of these experiments met or exceeded nutrient requirements for broilers recommended by the National Research Council( 13 ). The birds were allowed ad libitum access to feed and to tap water containing an undetectable amount of Zn in both experiments.
Table 1 Composition of two basal diets for 1–28-d-old broilers in Expt 1 (as-fed basis)
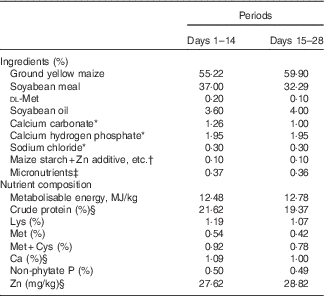
* Reagent grade.
† Zinc additive, lysine HCl or dl-methionine was added to the diets by replacing an equal weight of maize starch.
‡ Provided per kg of diet: for days 1–14 – vitamin A (all-trans retinol acetate), 4·46mg; vitamin D3 (cholecalciferol), 0·9mg; vitamin E (all-rac-α-tocopherol acetate), 33mg; vitamin K (menadione sodium bisulfite), 6 mg; thiamine (thiamine mononitrate), 4·5 mg; riboflavin, 10·5 mg; vitamin B6, 6 mg; vitamin B12, 0·03 mg; calcium pantothenate, 18 mg; niacin, 60 mg; folic acid, 1·8 mg; biotin, 0·165 mg; choline (choline chloride), 1000 mg; Cu (CuSO4.5H2O), 8 mg; Mn (MnSO4.H2O), 100 mg; Fe (FeSO4.7H2O), 80 mg; I (KI), 0·35 mg; Se (Na2SeO3), 0·15 mg; for days 15–28 – vitamin A (all-trans retinol acetate), 4·46mg; vitamin D3 (cholecalciferol), 0·9mg; vitamin E (all-rac-α-tocopherol acetate), 33mg; vitamin K (menadione sodium bisulfite), 6 mg; thiamine (thiamine mononitrate), 4·5 mg; riboflavin, 10·5 mg; vitamin B6, 6 mg; vitamin B12, 0·03 mg; calcium pantothenate, 18 mg; niacin, 60 mg; folic acid, 1·8 mg; biotin, 0·165 mg; choline (choline chloride), 700 mg; Cu (CuSO4.5H2O), 8 mg; Fe (FeSO4.7H2O), 80 mg; Mn (MnSO4.H2O), 100 mg; I (KI), 0·35 mg; Se (Na2SeO3), 0·15 mg.
§ Determined values.
Table 2 Composition of two diets for 1–28-d-old broilers in Expt 2 (as-fed basis)
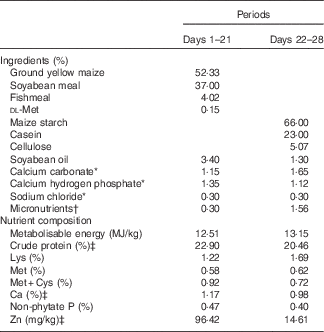
* Reagent grade.
† Provided per kg of diet: for days 1–21 – vitamin A (all-trans retinol acetate), 4·46mg; vitamin D3 (cholecalciferol), 0·9mg; vitamin E (all-rac-α-tocopherol acetate), 33mg; vitamin K (menadione sodium bisulfite), 6 mg; thiamine (thiamine mononitrate), 4·5 mg; riboflavin, 10·5 mg; vitamin B6, 6 mg; vitamin B12, 0·03 mg; calcium pantothenate, 18 mg; niacin, 60 mg; folic acid, 1·8 mg; biotin, 0·165 mg; choline (choline chloride), 1000 mg; Cu (CuSO4.5H2O), 8 mg; Zn (ZnSO4.7H2O), 60 mg; Mn (MnSO4.H2O), 100 mg; Fe (FeSO4.7H2O), 80 mg; I (KI), 0·35 mg; Se (Na2SeO3), 0·15 mg; for days 22–28 – vitamin A (all-trans retinol acetate), 4·46mg; vitamin D3 (cholecalciferol), 0·9mg; vitamin E (all-rac-α-tocopherol acetate), 33mg; vitamin K (menadione sodium bisulfite), 6 mg; thiamine (thiamine mononitrate), 4·5 mg; riboflavin, 10·5 mg; vitamin B6, 6 mg; vitamin B12, 0·03 mg; calcium pantothenate, 18 mg; niacin, 60 mg; folic acid, 1·8 mg; biotin, 0·165 mg; choline (choline chloride), 700 mg; K (KCl), 3000 mg; Mg (MgSO4.7H2O), 600 mg; Cu (CuSO4.5H2O), 8 mg; Fe (FeSO4.7H2O), 80 mg; Mn (MnSO4.H2O), 100 mg; I (KI), 0·35 mg; Se (Na2SeO3), 0·15 mg.
‡ Determined values.
Ligated loop procedure
In Expt 2, all 28-d-old birds were fasted overnight, and then weighed to equalise the body weights in all groups. The detailed in situ ligated duodenal surgical operation procedure was performed according to a previous protocol( Reference Yu, Lu and Luo 7 ). The solutions injected into the ligated duodenal loops were buffered with 15·5 mmol/l of morpholinoethanesulfonic acid at pH 6. Different Zn sources were added to the media to obtain the desired Zn concentrations. Phenol red was a non-absorbable marker used for correcting the changes in Zn concentration resulting from water absorption or intestinal secretion. The content of phenol red in perfusion solutions was 20 mg/l( Reference Yu, Lu and Luo 7 , Reference Schedl 14 ).
Sample collection and preparation
In Expt 1, on days 21 and 28, three birds per cage for each of the five treatments were selected according to the average body weight of the cage and anaesthetised by intravenous injections of Na pentobarbital (20 mg/kg body weight) via a wing vein. The blood sample was collected from the hepatic portal vein, and plasma was separated. The samples were pooled for each cage, resulting in eight composite samples per treatment, and then frozen (–20°C) for analysing the concentrations of Zn. After blood collection, one bird from each replicate cage for each treatment was killed and the intestinal mucosa was collected from three small intestinal segments of the bird as described by Bai et al.( Reference Bai, Lu and Wang 15 ), immediately frozen in liquid N2 and stored at –70°C for detecting the MT mRNA expression.
In Expt 2, an optimal sampling time of 30 min after the injection of solutions was used as established in our previous study( Reference Yu, Lu and Luo 7 ). Each treatment was repeated ten times using ten birds. The ligated duodenal loop of each bird was considered as one replication. A volume of 2 ml of perfusion solution was collected from the ligated duodenal loop of each bird and frozen (–20°C) for analysing the concentrations of Zn and phenol red. Then, all birds from the control and 0·616 mmol Zn/l groups were killed, and their ligated duodenal loops were immediately excised and rinsed with ice-cold saline, and then the intestinal mucosa was scraped from the underlying submucosa with a sterile glass slide. The mucosa samples were frozen in liquid N2 for detecting the mRNA expression levels of MT.
Determinations of zinc and phenol red concentrations
Zn concentrations in diets, plasma and perfusion solutions were determined by inductively coupled plasma emission spectroscopy (model IRIS Intrepid II; Thermo Jarrell Ash)( Reference Yu, Lu and Luo 7 ). The concentrations of phenol red in perfusion solutions were assayed by measuring absorbency at 520, 560 and 600 nm with a UV-Vis spectrophotometer (model Cary 100; Varian, Inc.)( Reference Steel and Cousins 16 ). Final volumes of solutions and absorption velocities of Zn were calculated according to the following equations:
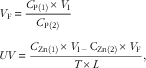 $$\eqalignno{ & V_{{\rm F}} \,{\equals}\,{{C_{{{\rm P}\left( 1 \right)}} {\times}V_{{\rm I}} } \over {C_{{{\rm P}\left( 2 \right)}} }} \cr & UV\,{\equals}\,{{C_{{{\rm Zn}\left( 1 \right)}} {\times}V_{{{\rm I}^\,{{\minus}\,} }} {\rm C}_{{{\rm Zn}\left( 2 \right)}} {\times}V_{{\rm F}} } \over {T{\times}L}}, $$
$$\eqalignno{ & V_{{\rm F}} \,{\equals}\,{{C_{{{\rm P}\left( 1 \right)}} {\times}V_{{\rm I}} } \over {C_{{{\rm P}\left( 2 \right)}} }} \cr & UV\,{\equals}\,{{C_{{{\rm Zn}\left( 1 \right)}} {\times}V_{{{\rm I}^\,{{\minus}\,} }} {\rm C}_{{{\rm Zn}\left( 2 \right)}} {\times}V_{{\rm F}} } \over {T{\times}L}}, $$
where V F is the final volume of perfusion solution (ml); C P(1) and C P(2) the initial and final concentrations (mg/l) of phenol red, respectively; V I the initial volume (ml) of injected dose; UV the absorption velocity of Zn (nmol/min per cm); C Zn(1) and C Zn(2) the Zn concentrations (mmol/l) of the initial and final perfusion solutions, respectively; T the sampling time (min) after initiation of dosing; and L the length (cm) of the ligated intestinal segment.
Quantitative real-time PCR procedure
In order to determine the mRNA expression levels of MT in intestinal mucosa samples by real-time PCR, MT-specific primers were designed using the software of Primer Premier 5.0, and the sequences were as follows: Forward 5'-AAG GGC TGT GTC TGC AAG GA-3', Reverse 5'-CTT CAT CGG TAT GGA AGG TAC AAA-3'. The protocols of RNA isolation, reverse transcription and real-time PCR were the same as those used in our previous study( Reference Yu, Lu and Luo 7 ).
Statistical analyses
The data were processed using Statistical Analysis Systems version 8.2 (SAS Institute). In Expt 1, analysis of the data was performed using two-way ANOVA with the general linear model. The model for plasma Zn content in the hepatic portal vein included the effects of Zn source, age and their interaction. The model for MT mRNA levels included the effects of Zn source, intestinal segment and their interaction. The replicate cage or individual chick served as the experimental unit. In Expt 2, the data of MT mRNA levels in the ligated duodenal loops perfused with solutions containing 0 (control) and 0·616 mmol Zn/l from one of the Zn sources were subjected to one-way ANOVA using the general linear model, with each loop as the experimental unit. The differences in kinetic parameters obtained from different Zn sources were analysed by Student’s t test. For all data, when ANOVA was significant, post hoc comparisons of treatment means were made using the least-squares mean test. Statistical significance was detected at P<0·05.
The kinetic analysis of Zn absorption was performed by fitting the data obtained from Expt 2 to the following equations: the first-order equation (a non-saturable diffusive component, Equation (1)), the Michaelis–Menten equation (a saturable process, Equation (2)) or two components including both equations mentioned above (a saturable process plus a non-saturable diffusive component, Equation (3))( Reference Condomina, Zornoza-Sabina and Granero 17 ):
where J Zn is the absorption velocity of Zn; J max is the maximum velocity in nmol/min per cm; Km is in mmol/l; P the diffusive constant in cm2/min and A the concentration of Zn in mmol/l.
The fits of experimental data to the equations were carried out using a non-linear least-square regression program (SigmaPlot version 9.0; SPSS Inc.). To select the best kinetic model of Zn absorption in this research, the Akaike information criterion (AIC)( Reference Gagne and Dayton 18 , Reference Akaike 19 ) has been adopted. The model with the smallest AIC was regarded as the ‘best’ model as it minimised the difference of the given model from the ‘true’ model. We have also considered the CV of the parameter obtained after each fit.
Results
Effect of four zinc sources on zinc content in plasma from the hepatic portal vein and body weights of broilers (Expt 1)
Significant differences (P<0·0001) among treatments were observed in plasma Zn content from the hepatic portal vein on days 21 and 28 of this experiment, but the effects of age (d) and treatment×age interaction were NS (P>0·14) (Table 3). On day 21, Zn content in plasma was increased (P<0·005) by 23·44 to 34·48 % for ZnSO4, Zn-AA W, Zn-Pro M and Zn-Pro S treatments, compared with the control, with no differences (P>0·15) among different Zn sources. However, Zn content in plasma was numerically 6·75–8·86 % higher for organic Zn sources with different chelation strength than for ZnSO4. On day 28, Zn content in plasma was increased (P<0·0001) by 31·94 to 42·93 % for ZnSO4, Zn-AA W, Zn-Pro M and Zn-Pro S treatments, compared with the control group. Plasma Zn content was 7·48 and 8·33 % higher (P<0·05) for Zn-Pro S than for Zn-AA W and ZnSO4, respectively. There were no differences (P>0·21) among ZnSO4, Zn-AA W and Zn-Pro M treatments. These findings showed that the absorption of Zn as Zn-Pro S was higher than that of Zn from ZnSO4, Zn-AA W and Zn-Pro M in the intestine of broilers. In addition, the mean body weights of these birds on either day 21 or day 28 were not affected (P>0·24) by the Zn sources, and were 758 (se 23), 745 (se 23), 741 (se 28), 759 (se 29) and 740 (se 29) g/bird on day 21, and 1021 (se 26), 1048 (se 29), 1025 (se 39), 1049 (se 29) and 1013 (se 36) g/bird on day 28 for the control, ZnSO4, Zn-AA W, Zn-Pro M and Zn-Pro S treatments, respectively.
Table 3 Effect of dietary zinc source on zinc content in plasma from the hepatic portal vein of 21- and 28-d-old chicks (Expt 1) (Mean values with their standard errors; n 8)
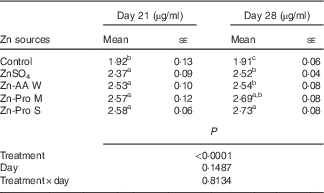
Zn-AA W, Zn-amino acid chelate with a weak chelation strength (Q f, 6·48); Zn-Pro M, Zn-protein chelate with a moderate chelation strength (Q f, 30·73); Zn-Pro S, Zn-protein chelate with a strong chelation strength (Q f 944·02).
a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·05).
mRNA expression levels of metallothionein in the duodenum, jejunum and ileum of intact living broilers as affected by four zinc sources (Expt 1)
The Zn source significantly affected (P<0·0001) MT mRNA expression levels in the duodenum, jejunum and ileum of 4-week-old broilers as shown in Fig. 1. Different Zn sources significantly up-regulated (P<0·0001) MT mRNA expression levels in three small intestinal segments of broilers compared with the control group without Zn addition. However, the differences among different Zn sources were not observed (P>0·27) in the duodenum and ileum. In the jejunum, MT mRNA expressions for Zn-AA W (P<0·0300) and ZnSO4 (P=0·0500) were significantly higher than those for Zn-Pro M, and there were no significant differences (P>0·46) among ZnSO4, Zn-AA W and Zn-Pro S, nor between Zn-AA M and Zn-Pro S. In addition, the changes of MT mRNA levels regulated by the same Zn source in different small intestinal segments were very sharp (P<0·0001). MT mRNA expressions in the ileum that were affected by different Zn sources were significantly lower (P<0·001) than those in the duodenum and jejunum, and sharply lower (P<0·003) in the jejunum than in the duodenum, except for no significant differences (P>0·16) between the jejunum and ileum in MT mRNA levels of the control and Zn-Pro M treatment. These results indicate that dietary supplementations with different Zn sources obviously increased MT mRNA expression levels in the duodenum, jejunum and ileum of broilers. In addition, the effect of Zn source on MT mRNA expressions in the jejunum was significant, and no differences were found in the other two small intestinal segments. Moreover, regardless of Zn source, MT mRNA expression levels were the highest in the duodenum, but the lowest in the ileum.

Fig. 1 Effect of zinc source on metallothionein (MT) mRNA expression levels in different small-intestinal segments of intact chicks (Expt 1). The zinc-deficient chicks (14-d old) were fed the basal diet (control (![]() ); containing about 28 mg zinc/kg) or the basal diet supplemented with 60 mg zinc/kg from either ZnSO4 (
); containing about 28 mg zinc/kg) or the basal diet supplemented with 60 mg zinc/kg from either ZnSO4 (![]() ), or one of three organic zinc sources with weak (Zn-AA W (
), or one of three organic zinc sources with weak (Zn-AA W (![]() ), Q
f 6·48), moderate (Zn-Pro M (
), Q
f 6·48), moderate (Zn-Pro M (![]() ), Q
f 30·73) and strong (Zn-Pro S (
), Q
f 30·73) and strong (Zn-Pro S (![]() ), Q
f 944·02) chelation strength for 14 d, respectively. MT mRNA levels were determined by real-time quantitative PCR. Data are presented in arbitrary units as relative mRNA abundances normalised to β-actin transcript abundance. Values are means (n 8), with their standard errors represented by vertical bars. Zinc source, intestinal segment and their interaction all had significant effects (P<0·0001). a,b,c Mean values with unlike superscript letters were significantly different (P<0·05) among different zinc sources for the same intestinal segment. A,B,C Mean values with unlike superscript letters were significantly different (P<0·05) among different intestinal segments for the same source.
), Q
f 944·02) chelation strength for 14 d, respectively. MT mRNA levels were determined by real-time quantitative PCR. Data are presented in arbitrary units as relative mRNA abundances normalised to β-actin transcript abundance. Values are means (n 8), with their standard errors represented by vertical bars. Zinc source, intestinal segment and their interaction all had significant effects (P<0·0001). a,b,c Mean values with unlike superscript letters were significantly different (P<0·05) among different zinc sources for the same intestinal segment. A,B,C Mean values with unlike superscript letters were significantly different (P<0·05) among different intestinal segments for the same source.
Kinetic absorption of organic and inorganic zinc in the ligated duodenum (Expt 2)
The kinetic Zn absorption of different Zn sources was investigated within a wide Zn concentration range (Fig. 2). We found that Zn absorption was improved with increasing Zn concentrations regardless of Zn source. The regression analyses showed that the best fits for Zn absorption from different Zn sources in ligated duodenal loops were to Equation (2). It means that organic Zn absorption is a saturable carrier-mediated process in duodenal loops, which is similar to the inorganic Zn absorption process. The kinetic parameters obtained, J max and Km, are outlined in Table 4. The Km values were higher for Zn-AA W, Zn-Pro M and Zn-Pro S than for the ZnSO4 (6·85, 7·14 and 7·57 v 4·53 mmol/l), but the differences were NS (P>0·05). The different Km values demonstrate that the carriers involved in regulating Zn absorption from different Zn sources may be different, because different carriers have different affinities with Zn. The J max values were obviously greater(P<0·01) for the Zn-AA W, Zn-Pro M and Zn-Pro S than for the ZnSO4 (22·01, 30·47 and 30·74 v. 11·39 nmol/min per cm), with no differences (P>0·38) among organic Zn sources, suggesting that Zn absorption from organic Zn sources might be higher than that from the inorganic Zn source. In addition, organic Zn sources with greater Q f values tended to have a higher Zn absorption.

Fig. 2 Kinetic absorption of zinc from different zinc sources in the ligated duodenal loops of zinc-deficient chicks (Expt 2). The ligated duodenal loops were perfused with solutions containing 0·077–2·464 mmol zinc/l from either (a) ZnSO4, or one of three organic zinc sources with (b) weak (Zn-AA W, Q f 6·48), (c) moderate (Zn-Pro M, Q f 30·73) and (d) strong (Zn-Pro S, Q f 944·02) chelation strength, respectively. At 30 min after perfusion, zinc transport (disappearance of zinc from the ligated duodenal loop) was determined and the initial rate of zinc transport was calculated. Values of zinc transport rates are means (n 8), and standard deviations represented by vertical bars. All kinetic curves of zinc transport from different zinc sources in the duodenum are described by the Michaelis–Menten equation (a saturable process).
Table 4 Kinetic and statistical parameters obtained after fitting Michaelis–Menten equations to the experimental data of zinc uptake in the ligated duodenal loops of chicks (Expt 2) (Mean values with their standard errors; n 8)
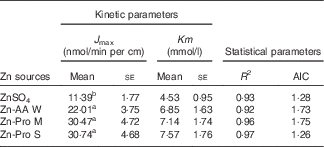
J max, maximum absorption velocity of Zn; AIC, Akaike information criterion; Zn-AA W, Zn-amino acid chelate with a weak chelation strength (Q f, 6·48); Zn-Pro M, Zn-protein chelate with a moderate chelation strength (Q f, 30·73); Zn-Pro S, Zn-protein chelate with a strong chelation strength (Q f, 944·02).
a,b Mean values within a column with unlike superscript letters were significantly different (P<0·05).
mRNA expression levels of metallothionein in the ligated duodenum as affected by four zinc sources (Expt 2)
The MT mRNA expression levels in the ligated duodenum of 4-week-old broilers were significantly affected (P<0·05) by the Zn source as shown in Fig. 3. Compared with the control group, the perfusions of organic Zn sources significantly up-regulated (P<0·05) MT mRNA levels in the ligated duodenum, and they were about 1·5–2·1 times of those in the control group. The MT mRNA levels were significantly higher (P<0·04) for the Zn-Pro S group than for the Zn-Pro M and ZnSO4 groups, and for the Zn-AA W group than for the ZnSO4 group. However, no differences (P>0·22) were observed between the control and ZnSO4 groups, the ZnSO4 and Zn-Pro M groups, the Zn-AA W and Zn-Pro M groups or the Zn-AA W and Zn-Pro S groups. These data demonstrate that organic Zn sources, especially Zn-Pro S, boosted MT transcriptional expressions in the duodenum of broilers.

Fig. 3 Effect of zinc source on metallothionein (MT) mRNA expression levels in the ligated duodenal loops of zinc-deficient chicks at 30 min after perfusion as determined by real-time quantitative PCR (Expt 2). The treatments included a zinc-free basal solution (control) and the basal solution supplemented with 0·616 mmol zinc/l (close to the dietary requirement of 90 mg zinc/kg for broilers) from either ZnSO4, or one of three organic zinc sources with weak (Zn-AA W, Q f 6·48), moderate (Zn-Pro M, Q f 30·73) and strong (Zn-Pro S, Q f 944·02) chelation strength, respectively. Data are presented in arbitrary units as relative mRNA abundances normalised to β-actin transcript abundance. Values are means (n 8), with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike superscript letters were significantly different (P<0·05) among different zinc sources.
Discussion
Zn is absorbed from intestinal-mucosal cells and subsequently transported to the portal blood of the liver, and albumin is the main plasma carrier that is involved in this removal process( Reference Smith, Failla and Cousins 20 , Reference Vallee and Falchuk 21 ). Thus, Zn content in plasma of the hepatic portal vein can accurately reflect the differences in Zn absorption from different Zn sources. We investigated the effect of different Zn sources on Zn content in plasma of the hepatic portal vein using intact living broilers, and found that the absorption of Zn from organic Zn sources was higher than that of Zn from the inorganic Zn source, and the absorption of Zn from the Zn-Pro S was higher than that of Zn from ZnSO4, Zn-AA W and Zn-Pro M. These results are similar to our previous findings on Mn content in plasma from the hepatic portal vein of broilers, in which the absorption of Mn from the organic Mn source with strong chelation strength was higher than that of Mn from either MnSO4 or the organic Mn source with weak or moderate strength in broilers( Reference Ji, Luo and Lu 22 ). In addition, we have previously reported that the absorption of Zn as organic Zn was more effective than that of Zn as inorganic Zn( Reference Yu, Lu and Wang 8 ). Organic Zn absorption increased with increasing chelation strength as well. Moreover, organic Zn sources could lessen the negative effect of phytate on Zn absorption; further, the absorption of organic Zn, especially of Zn-Pro S, was more effective than that of inorganic Zn under a high level of phytate( Reference Yu, Lu and Wang 8 ). The data acquired using intact living broilers in the present study confirmed the above results obtained from in situ ligated small intestinal loops of broilers.
There are two assumptions about the absorption mechanisms of mineral complexes. One is that ‘complexed’ or ‘chelated’ trace minerals are absorbed in their intact form, and the metal atoms remain safely bound or protected within organic molecular structures or ‘ligands’ during absorption. The other is that organic ligands can prevent the harmful effect of competitive ligands such as phosphate, phytate and other compounds, which can bind free metal ions and render the minerals unavailable for absorption. Maintaining a dietary mineral in solution allows maximum opportunity for contact with intestinal mucosa, and then Zn from a Zn–ligand complex or chelate may undergo dissociation to a high-affinity site on the brush-border membrane( Reference Ashmead 23 , Reference Oestreicher and Cousins 24 ). Koike et al.( Reference Koike, Kratzer and Vohra 25 ) suggest that the Zn–EDTA complex be transported from the chicken lumen to the plasma intactly because the content of 65Zn in plasma is equal to that of 14C. This is in agreement with the work of Suso & Edwards( Reference Suso and Edwards 26 ) and Hempe & Cousins( Reference Hempe and Cousins 27 ). Wapnir et al.( Reference Wapnir, Khani and Bayne 28 ) found that the Zn–l-histidine complex could be absorbed in its intact form by perfused rat intestines in the same way as histidine. Lowe et al.( Reference Lowe, Wiseman and Cole 29 ) studied the absorption and retention of Zn administered as ZnMet chelate in dogs, and found that the coefficients determined for absorption were closer to those determined for the absorption of amino acids, thus supporting the fact that Zn as an amino acid chelate is transported across the intestinal enterocyte and into the circulation in an intact form. However, there are many reports providing evidence to support the second assumption that organic Zn could be absorbed as Zn ions. Hill et al.( Reference Hill, Peo and Lewis 2 , Reference Hill, Peo and Lewis 3 ) demonstrated that the 14C, 3H and 65Zn content in the everted rat gut, which were used to mark 65ZnMet (14C) and 65ZnLys (3H) chelates, were not equal. Therefore, the Zn–amino acid complexes or chelates do not appear to be absorbed intactly, and organic Zn may be dissociated into Zn ions in the brush-border membrane before absorption. This conclusion was similar to the work of Beutler et al.( Reference Beutler, Pankewycz and Brautigan 5 ) who found that when equimolar amounts of 65Zn and [35S]methionine were added to cells, the uptake of 65Zn was >1000 times the uptake of [35S]methionine. The data support the concept that ZnMet dissociates in solution, and that the metal ion and amino acid are taken up separately. Our previous data demonstrated that Zn-Pro S was the least available, suggesting that it may be difficult to dissociate Zn from Zn-Pro S and mobilise it for metabolic utilisation by the body tissue( Reference Huang, Lu and Li 30 , Reference Huang, Lu and Xie 31 ). In the present study, the absorption of Zn as Zn-Pro S was the highest among different Zn sources. These findings show that Zn absorption as Zn-Pro S may be in line with the first hypothesis that organic Zn sources are absorbed in an intact form.
In addition, we investigated the kinetic curve of organic Zn absorption in the ligated duodenum of the broiler, and found that the patterns of Zn absorption from the four different Zn sources all followed the same saturable carrier-mediated process in the duodenum, which is in agreement with the methods of inorganic Zn absorption reported in our previous study( Reference Yu, Lu and Luo 7 ). Even so, the J max values of organic Zn sources with different chelation strength were significantly higher than those of inorganic Zn sources, and they were 22·01 (Zn-AA W), 30·47 (Zn-Pro M), 30·74 (Zn-Pro S) and 11·39 (ZnSO4) nmol/min per cm, respectively, further indicating that the absorption of organic Zn was better than that of inorganic Zn. In addition, there was a decreased trend of the Km value for ZnSO4 compared with that for organic Zn sources, suggesting that the carriers involved in organic Zn absorption might have a higher affinity for Zn, and this may be the partial reason for organic Zn absorption being better than inorganic Zn absorption.
MT is a small, cysteine-rich protein that is especially prevalent in the liver, kidney and intestine. Evidence shows that high ambient concentrations of Zn could increase MT expression, which can combine and store Zn, or increase the secretion of Zn from the small intestine to the lumen, and then reduce the absorption of Zn( Reference Davis, McMahon and Cousins 10 , Reference Smith and Cousins 32 – Reference Hempe, Carlson and Cousins 35 ). However, other researchers reported that MT content in the intestines of mice had no effect on Zn absorption by administering, gavaging or perfusing Zn( Reference Flanagan, Haist and Valberg 36 , Reference Olafson 37 ). In our present study, Zn supplementation, regardless of the Zn source, significantly increased MT mRNA expression levels in three small intestinal segments of broilers, as seen in Expt 1, which is in agreement with previous data in other animals( Reference Huang, Lu and Luo 12 , Reference Rojas, McDowell and Cousins 38 – Reference Sandoval, Henry and Luo 40 ). Moreover, MT mRNA levels in the duodenum were higher for organic Zn (Zn-AA W and Zn-Pro S) than for inorganic Zn in Expt 2. Cao et al.( Reference Cao, Henry and Davis 41 ) also showed that organic Zn sources with moderate or strong complex strength slightly up-regulated the MT protein level in the intestine of broilers, compared with the inorganic Zn source. However, Carlson( Reference Carlson 42 ) demonstrated that ZnMet added to the diet significantly decreased MT content in the intestinal mucosa of pigs, compared with ZnO. The above disparities might be caused by different experimental animals or Zn sources used in these studies. In addition, these data are inconsistent with those of Expt 1 which showed that no differences in the duodenal MT mRNA were found among the four different Zn sources. The different methods of Zn administration (dietary supplementation v. duodenal perfusion) in Expt 1 and Expt 2 might partially explain the inconsistency. Importantly, when MT mRNA levels in the duodenum were higher for the organic Zn than for the inorganic Zn, then it could not be elucidated why the organic Zn absorption was better than that of the inorganic Zn because of the above reports indicating that increasing MT expression could reduce the absorption of Zn. However, Pekarek & Evans( Reference Pekarek and Evans 43 , Reference Pekarek and Evans 44 ) found that bacterial infection with lipopolysaccharide promoted the absorption of Zn, while intestinal MT expression also increased, which is in line with our findings. Starcher et al.( Reference Starcher, Glauber and Madaras 45 ) obtained similar results. In recent years, a number of mammalian Zn transporters have been molecularly characterised. This has brought about major advances in our understanding of the tight regulation of cellular Zn homoeostasis and the pivotal roles which Zn transporters play in a variety of biological events( Reference Lichten and Cousins 46 , Reference Kambe 47 ). Therefore, other Zn transporters, except for MT, might have also participated in the absorption of Zn from different Zn sources; further efforts are needed to characterise these proteins in future studies.
In summary, our results further confirm that organic Zn absorption is more effective than that of inorganic Zn, and Zn absorption from organic Zn sources with strong chelation strength was higher in intact living broilers; organic Zn absorption in the ligated duodenal segment is a saturable carrier-mediated process similar to that of ZnSO4. Moreover, except for MT, there may be other Zn transporters involved in Zn absorption that are affected by different Zn sources.
Acknowledgements
The present study was supported by the Key International Cooperation Program of the National Natural Science Foundation of China (project no. 31110103916; Beijing, People’s Republic of China), the Research Program of the Key Laboratory of Animal Nutrition (project no. 2004DA125184G1108; Beijing, PR China), the Agricultural Science and Technology Innovation Program (ASTIP-IAS08; Beijing, PR China), the China Agriculture Research System (project no. CARS-42; Beijing, PR China) and the Program of the National Natural Science Foundation of China (project no. 30871798; Beijing, PR China).
Y. Y. and X.-G. L. conceived and designed the research and revised the manuscript; Y. Y., L. L., S.-F. L. and L.-Y. Z. performed the experiments; Y. Y., L. L. and S.-F. L. analysed the data; Y. Y. wrote the manuscript.
None of the authors has any conflicts of interest to declare.










