CLINICIAN'S CAPSULE
What is known about the topic?
Published studies demonstrate a wide range of sensitivities and specificities for the various components of the eFAST scan in trauma.
What did this study ask?
Through a systematic review process, we examined the pooled sensitivities and specificities for the components of an eFAST exam.
What did this study find?
The eFAST exam in trauma is helpful to rule in, but not to rule out, pneumothorax, pericardial effusion, and intra-abdominal free fluid.
Why does this study matter to clinicians?
An eFAST scan is an accepted part of the trauma assessment, and users should know the strengths and limitations of the test.
BACKGROUND
Traumatic injuries are the most common cause of morbidity and premature mortality in young adults, and the incidence of trauma presentations with a high injury severity is increasing over time.Reference Dutton, Stansbury and Leone1–Reference Berwick, Downey and Cornett3
The extended Focused Assessment with Sonography in Trauma (eFAST) exam is an accepted part of the trauma assessmentReference Kirkpatrick, Sirois and Laupland4,Reference Abdulrahman, Musthafa and Hakim5 and can be used to identify pneumothorax (PTX), pericardial effusions (PCE), and intra-abdominal free fluid (FF).Reference Rippey and Royse6 Early detection of these findings can help clinicians prioritize the performance of further diagnostic and therapeutic interventions.Reference Rippey and Royse6,Reference Rose7
Multiple studies have examined the use of ultrasound in the trauma setting, with variable reported sensitivities and specificities.Reference Alrajhi, Woo and Vaillancourt8,Reference Nishijima, Simel, Wisner and Holmes9 Prior reviews have used dated gold standards,Reference Wilkerson and Stone10 limited ultrasound use to surgeons,Reference Beggs and Thomas11 or studied components of the eFAST in the pre-hospital setting.Reference Jørgensen, Jensen and Dirks12,Reference O'Dochartaigh and Douma13 Two recent reviewsReference Staub, Biscaro, Kaszubowski and Maurici14,Reference Stengel, Bauwens and Sehouli15 examined the accuracy of an ultrasound for a patient with trauma, but no comprehensive systematic review has been performed examining the accuracy of all components of the eFAST exam. As such, we sought to determine the diagnostic accuracy of the eFAST exam for the detection of PTX, PCE, and FF in the undifferentiated trauma patient.
METHODS
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) while undertaking this review.Reference Liberati, Altman and Tetzlaff16
Search strategy
In collaboration with an expert librarian at the University of Saskatchewan, a search strategy was developed to search Embase and MEDLINE since inception (see Appendix One). Search terms (medical subject, Emtree headings, and free text words) related to trauma patients; bedside ultrasound; and the detection of PTX, PCE, and FF were combined using Boolean Logic. The search was initially performed on August 29, 2017, and was updated on October 19, 2018. No age or language restrictions were applied.
Study selection
Search results were combined using Covidence™ Software (www.covidence.org), and duplicates were removed. Titles and abstracts were initially screened for inclusion by two independent reviewers (SN and VM) prior to a full-text review. Bibliographies of all included studies were also reviewed.
Our population of interest was trauma patients (blunt, penetrating, or polytrauma), who were assessed in an emergency department (ED) or trauma centre, underwent an ultrasound examination during their initial assessment, and subsequently had a gold standard test performed. The ultrasound was considered positive for PTX if a lung point or lack of a lung slide was seen. Hypoechoic fluid in the appropriate anatomic location was considered a positive ultrasound scan for PCE or FF. These definitions of positive scans were consistent across the included studies. Pelvic assessments for FF were not included. The gold standard comparator for PTX was a computed tomography (CT) scan or gush of air with chest tube insertion. For FF, the gold standard was positive laparotomy findings, diagnostic peritoneal lavage/aspirate (DPL/DPA), or CT scan; for PCE, it was a CT scan or positive intra-operative findings. Disagreements regarding study inclusion were resolved by consensus, and, if consensus could not be achieved, a third independent reviewer (PD) adjudicated. For studies published in a foreign language, study authors were contacted for an English translation of their work, and, if not available, a translation attempt was made using an online translation program (Google Translate™).
Outcomes of interest
The primary outcome of interest was the sensitivity and specificity of eFAST. For inclusion, studies were required to have sensitivities and specificities expressed in a 2 × 2 table or to have provided enough information for the creation of a 2 × 2 table. Studies were excluded if they were performed in the wrong setting (pre-hospital), involved the wrong population (non-trauma), did not have an abovementioned gold standard comparator, or had incomplete data. All study designs were included except case reports and case series.
A subgroup analysis was planned for pregnant, geriatric, pediatric, and hypotensive trauma patients. Because of a paucity of available literature, the subgroup analysis was only possible for FF in pediatric and hypotensive patients. Because of high heterogeneity, a subgroup analysis of FF in adult normotensive patients was also performed.
Critical appraisal of included studies
Risk of bias (ROB) was evaluated by two independent reviewers (SN and VM) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS 2) tool.Reference Whiting, Rutjes and Westwood17 This tool examines the ROB in four domains: 1) patient selection; 2) the index test (ultrasound); 3) the reference standards (appropriate gold standard); and 4) timing and flow. Please see Appendix 2 for further information on the ROB domains and applicability concerns for all included studies, as well as the prompting questions used for this analysis.
Data extraction
Data were extracted using a piloted data extraction form by two independent reviewers (SN and VM). If discrepancies arose, an attempt at consensus was made, and, if consensus could not be reached, a third reviewer (PD) adjudicated. Extracted information included: gold standard test, study design, patient characteristics (age and gender), type of trauma, true positives, true negatives, false positives, and false negatives for an ultrasound while investigating for the presence of PTX, PCE, or FF.
Data synthesis
Data were compiled using Meta-DiSc™ softwareReference Zamora, Abraira, Muriel, Khan and Coomarasamy18 and subsequently analyzed in collaboration with a statistician at the Clinical Research Support Unit (CRSU) at the University of Saskatchewan. A bivariate random effect model was used for generating pooled sensitivities, specificities, and positive and negative likelihood ratios.
RESULTS
After removing duplicates, our search strategy yielded 767 articles. After a title and abstract screen, 119 publications underwent a full-text review, of which 71 papers met the inclusion criteria. An additional four papers were added from the bibliographic review, representing a total of 75 included studies (Appendix 3). Seventeen of the included studies examined the eFAST detection of PTX (Appendix 4), nine studies considered the detection of PCE (Appendix 5), and 52 examined the detection of FF (Appendix 6).
PTX
Table 1 summarizes the patient characteristics and study outcomes of the 17 included studies examining the use of ultrasound for the identification of PTX. These studies included a total of 3653 patients. Five of the studies (as indicated in Table 1) considered each lung as a data point, meaning that each patient provided up to two data points. All studies combined provided 4816 data points. The average age was 39.8 years, 75% of the patients were male, and the predominating injury occurred through a blunt mechanism. Pooled sensitivity (Figure 1a) was 0.694 (95% confidence interval [CI] 0.660–0.727; I2 = 91%), and pooled specificity (Figure 1b) was 0.99 (95% CI 0.99–0.99; I2 = 66.9%), with high heterogeneity amongst included studies (see Figure 1a and b). The area under the curve (AUC) for the summary receiver operating characteristic (sROC) curve was 0.994 (Appendix 7), and the pooled accuracy was 0.943. Appendix 10 summarizes the ROB for the included studies. Overall, the ROB for the studies investigating PTX was low to moderate, with all bias largely coming from patient selection (Kappa values, as indicated in Appendix 3).
Table 1. Patient characteristics and study outcomes of the 17 included studies examining the use of ultrasound for identification of PTX.
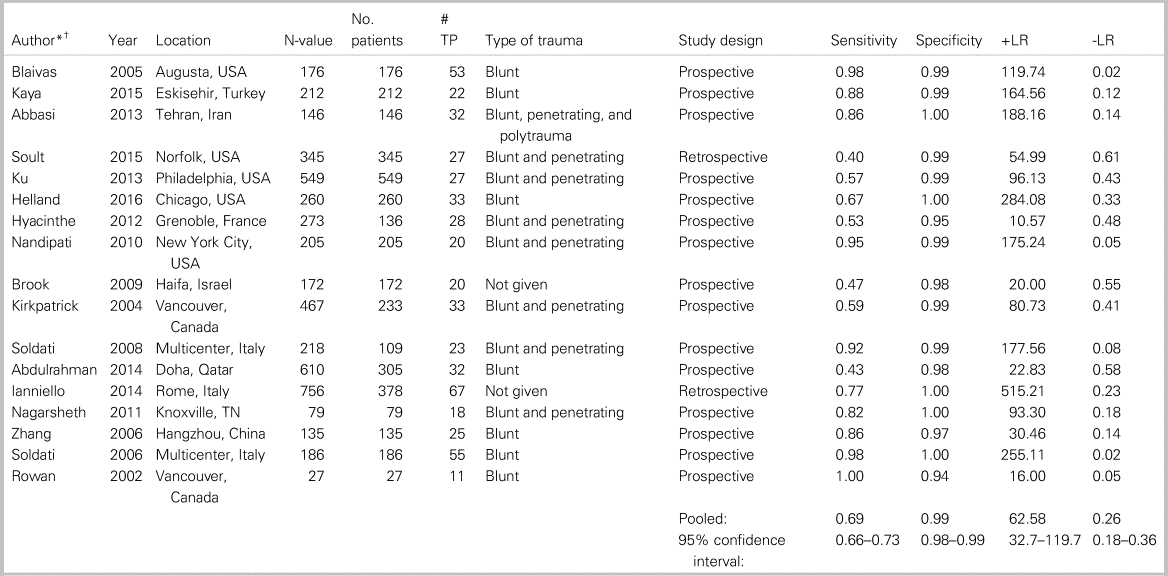
-LR = negative likelihood ratio; +LR = positive likelihood ratio; TP = true positives.
* Studies by Hyacinthe, Kirkpatrick, Soldati, Abdulrahman, and Ianniello included each lung scanned as an n-value, but the other studies counted each patient scanned as an n-value.
† See Appendix 4 for a list of references.
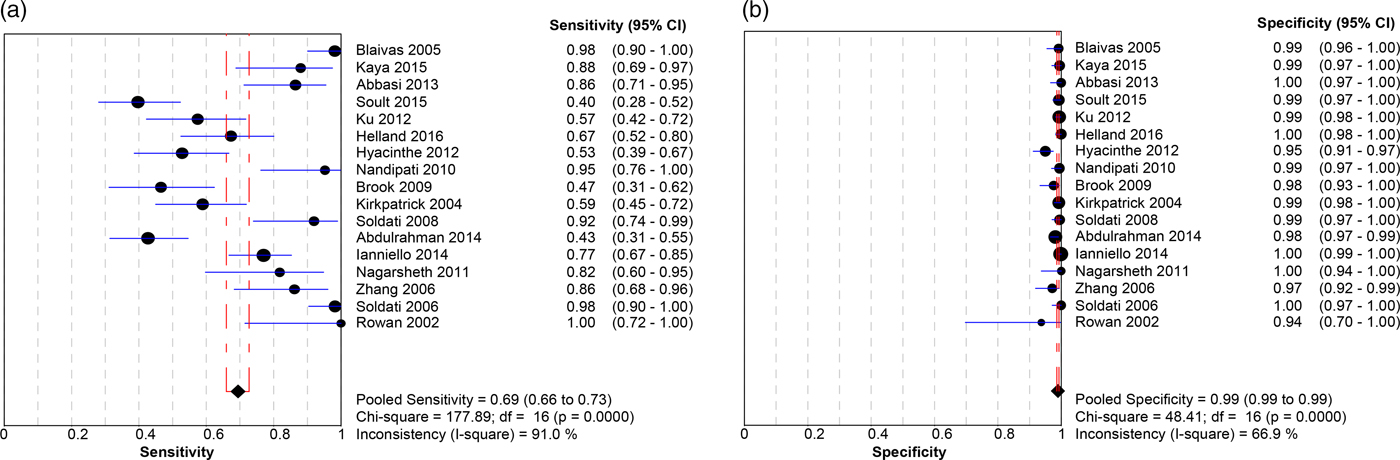
Figure 1. Forest plot displaying the sensitivity (a) and specificity (b) of the included studies for ultrasound identification of PTX in trauma patients.
PCE
Table 2 summarizes patient characteristics and study outcomes from the nine studies examining the use of ultrasound for identification of PCE. These studies included a total of 1,031 patients and included only penetrating trauma. The average patient age was 30 years, with a male predominance (86%). Appendix 10 summarizes the ROB and concerns for applicability for all included studies. These studies averaged a low to moderate ROB, with all bias arising solely from factors involved with patient selection (Kappa values, as indicated in Appendix 3). The pooled sensitivity (Figure 2a) was 0.912 (95% CI 0.870–0.944; I2 = 65.6%), and pooled specificity (Figure 2b) was 0.941 (95% CI 0.922–0.957; I2 = 96.6%), with high heterogeneity amongst the included studies (see Figure 2a and b). The AUC of the sROC curve (Appendix 8) was 0.975, and the pooled accuracy was 0.934. There were two studies that presented significant outlying results.Reference Nicol, Navsaria, Beningfield, Hommes and Kahn19,Reference Boulanger, Kearney, Tsuei and Ochoa20 Further, a sensitivity analysis with these two studies removed yielded a sensitivity of 0.982 (95% CI 0.937–0.998; I2 = 0%) and specificity of 0.985 (95% CI 0.973–0.992; I2 = 66.8%).
Table 2. Patient characteristics and study outcomes from the nine studies examining the use of ultrasound for identification of a PCE.
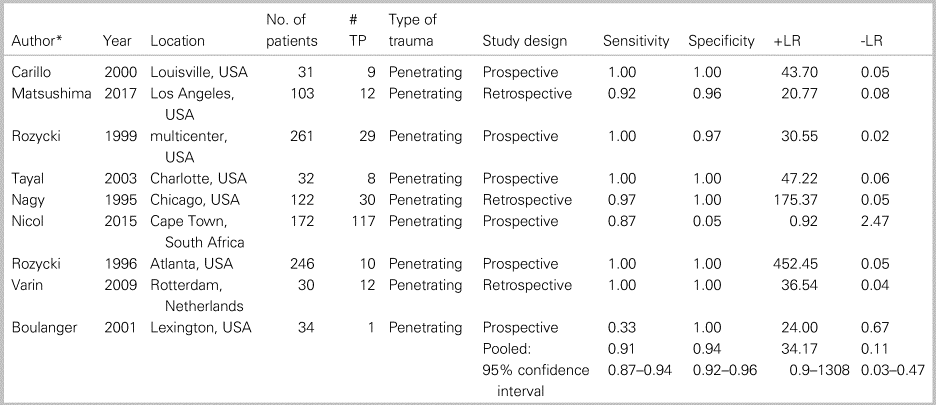
-LR = negative likelihood ratio; +LR = positive likelihood ratio; TP = true positives.
* See Appendix 5 for references.

Figure 2. Forest plot displaying the sensitivity (a) and specificity (b) of the included studies for ultrasound identification of PCE in trauma patients.
Intra-abdominal FF
Table 3 summarizes the patient characteristics and study outcomes of the 52 included studies examining the use of ultrasound for the identification of FF. These studies included a total of 19,666 patients, with an average age of 33.3 years, of whom 68.4% were male. One studyReference Goodwin, Holmes and Wisner21 examined only pregnant trauma patients, of whom 46%, were in their third trimester. Six studies included only pediatric patients.Reference Corbett, Andrews, Baker and Jones22–Reference Thourani, Pettitt, Schmidt, Cooper and Rozycki27 Eight additional studies included patients of all ages (pediatric and adult) but did not stratify patients based on age.Reference Holmes, Harris and Battistella28–Reference Cheung, Wong, Leung, Tsang and Leung35 Five papers included only hypotensive patients (systolic blood pressure [sBP] of <90 mm Hg).Reference Holmes, Harris and Battistella28,Reference Matsushima, Khor and Berona36–Reference Lentz, McKenney and Nuñez39 One studyReference Kirkpatrick, Sirois and Laupland40 compared both a hand-held ultrasound machine and a regular ultrasound machine against the gold standard, providing two sets of data from one paper.
Table 3. Patient characteristics and study outcomes of the 52 included studies examining the use of ultrasound for identification of intra-abdominal FF
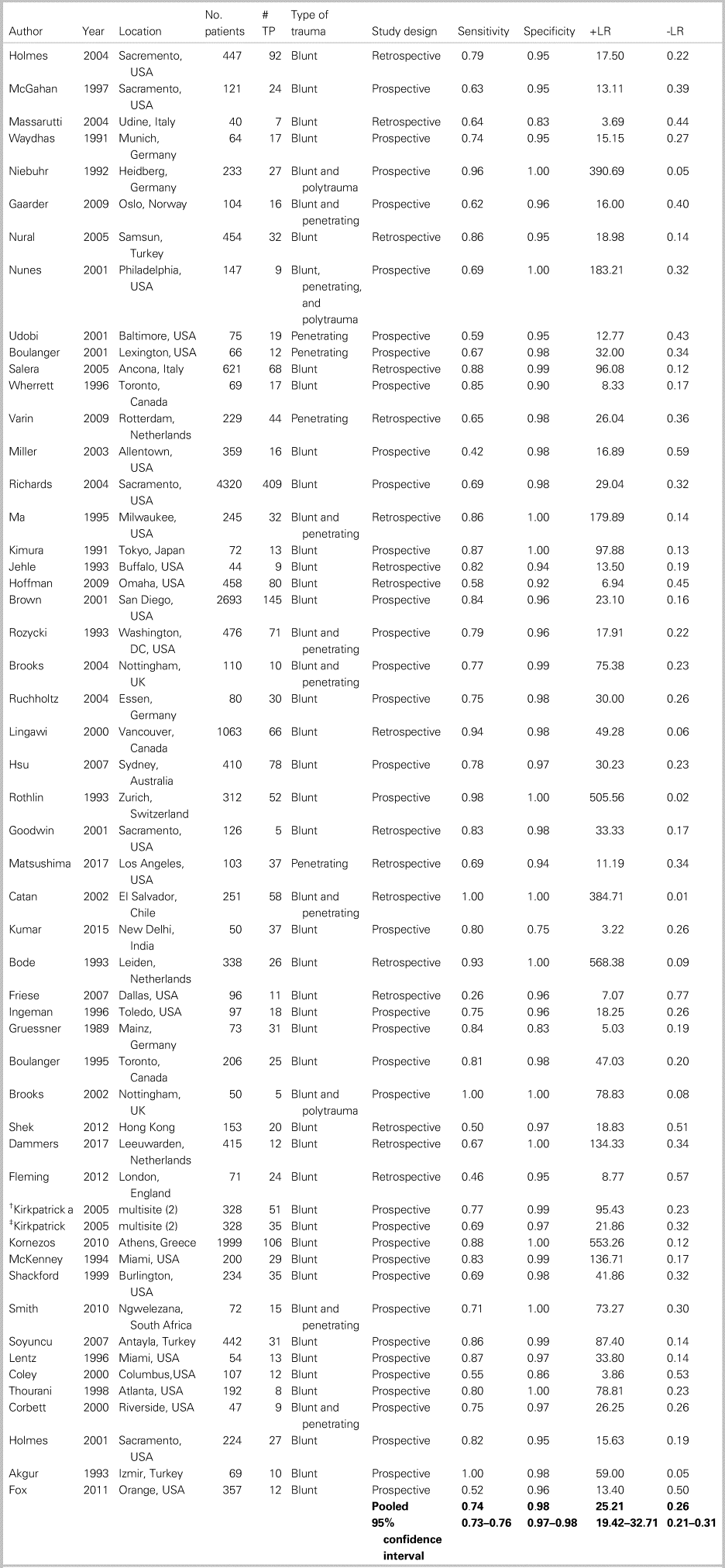
-LR = negative likelihood ratio; +LR = positive likelihood ratio; TP = true positives.
*See appendix 6 for references.
† Kirkpatrick = handheld ultrasound.
‡ Kirkpatrick = regular ultrasound.
Appendix 10 summarizes the ROB for all included studies. Overall, the ROB was low to moderate. Both patient selection and flow and timing factored into the overall bias assessment of these papers (Kappa values, as indicated in Appendix 3). The pooled sensitivity (Figure 3a) was 0.742 (95% CI 0.726–0.758; I2 = 82.7%), and the pooled specificity (Figure 3b) was 0.976 (95% CI 0.973–0.978; I2 = 83%), with high heterogeneity amongst the included studies (see Figure 3a and b). The AUC of the sROC curve (Appendix 9) was 0.931, and the overall pooled accuracy was 0.942.
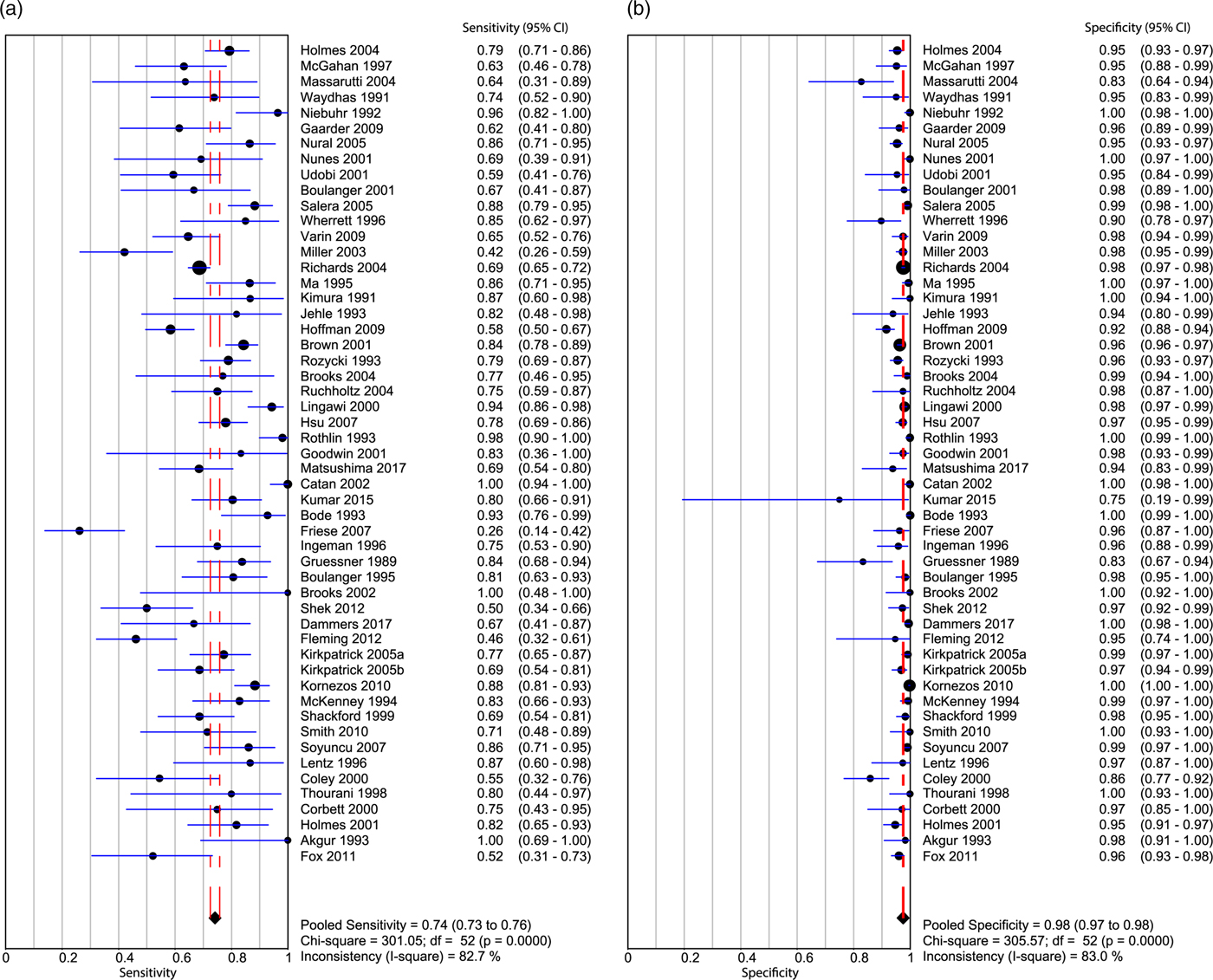
Figure 3. Forest plot displaying the sensitivity (a) and specificity (b) of the included studies for ultrasound identification of intra-abdominal FF in trauma patients.
If only pediatric patients were considered, the pooled sensitivity was 0.709 (95% CI 0.615–0.792; I2 = 68.1%) and the pooled specificity was 0.951 (95% CI 0.933–0.965; I2 = 72.1%).Reference Corbett, Andrews, Baker and Jones22–Reference Thourani, Pettitt, Schmidt, Cooper and Rozycki27 The pooled positive likelihood ratio was 14.13 (95% CI 6.533–30.567; I2 = 70.9%). The pooled negative likelihood ratio was 0.32 (95% CI 0.193–0.535; I2 = 62.6%). The AUC of the sROC curve was 0.959, and the pooled accuracy from these studies was calculated to be 0.92.
Among studies examining only hypotensive patients (sBP < 90 mm Hg),Reference Holmes, Harris and Battistella28,Reference Matsushima, Khor and Berona36–Reference Lentz, McKenney and Nuñez39 and the pooled sensitivity was 0.743 (95% CI 0.681–0.799; I2 = 38.7%). The pooled specificity was 0.949 (95% CI 0.926–0.966; I2 = 41.5%). The pooled positive likelihood ratio was 11.87 (95% CI 5.864–24.056; I2 = 60.1%). The pooled negative likelihood ratio was 0.30 (95% CI 0.214–0.423; I2 = 48.7%). The AUC of the sROC curve was 0.856, and the pooled accuracy from these studies was calculated to be 0.888.
Among studies examining only adult normotensive patients, the pooled sensitivity was 0.76 (95% CI 0.739–0.781; I2 = 84.7). The pooled specificity was 0.98 (95% CI 0.975–0.981; I2 = 85.4%). The pooled positive likelihood ratio was 33.5 (95% CI 23.354–48.121; I2 = 80.1%), and the pooled negative likelihood ratio was 0.231 (95% CI 0.176–0.304; I2 = 90.3%). The AUC of the sROC curve was 0.946, and the pooled accuracy from these studies was calculated to be 0.949.
DISCUSSION
The undifferentiated trauma patient can present several simultaneous diagnostic and disposition challenges. The eFAST exam provides trauma practitioners with a bedside tool that can provide adjunctive information to the primary survey and help prioritize care.
Two recent reviews evaluated components of the eFAST exam, and although methodologically different from this review, they reach similar conclusions.Reference Staub, Biscaro, Kaszubowski and Maurici14,Reference Stengel, Bauwens and Sehouli15 Staub et al. investigated detection of both PTX and hemothorax (which was not a focus of this review) and reported a sensitivity of 81%, a specificity of 98%, and an AUC of 0.979 for ultrasound detection of PTX that is very similar to the one presented here (0.994, Appendix 7).
Stengel et al.Reference Stengel, Bauwens and Sehouli15 examined the use of ultrasound in blunt thoracoabdominal trauma patients. Their analysis had two subgroups: 1) all abdominal injury (FF, organ injury, vascular injury; sensitivity 68% and specificity 95%); or 2) abdominal FF and/or intra-abdominal free air (sensitivity 78% and specificity 97%). Direct comparison to our review is difficult as we did not look at ultrasound use for the detection of free intra-abdominal air, organ injury, or vascular injury. In their thoracic group analysis, only four papers investigating the use of ultrasound in the detection of PTX's were included, making direct comparison difficult. Regardless of these differences, the reported sensitivities and specificities are very similar to our findings.
Identification of pneumothorax
Our results suggest a moderate sensitivity and good specificity in detecting PTX in the trauma setting, corresponding to a positive likelihood ratio of 62.57 and a negative likelihood ratio of 0.256 (Table 1). While our results suggest that the eFAST scan can be used as a rule-in test for the detection of PT, it lacks adequate sensitivity to be used as a rule-out test. Further, it should be noted, that depending on the patient's age and comorbidities, several false positives (e.g., prior pleurodesis and interstitial lung disease) and negatives (e.g., small PTX, and subcutaneous air) can occur.Reference Volpicelli41
A previous review from 2012 included eight studies examining ultrasound detection of PTX in traumatic and non-traumatic patients and suggested a similar specificity (98.2%), with a much higher sensitivity of 90.9%, however with significant heterogeneity.Reference Alrajhi, Woo and Vaillancourt8 This discrepancy could be explained by technological improvements in the gold standard test and the increased frequency in the detection of occult pneumothoraces because of the increased use of CT.
A more recent 2018 systematic found a sensitivity of 81% and specificity of 98% in ultrasound detection of traumatic PTX.Reference Staub, Biscaro, Kaszubowski and Maurici14 For various reasons, our review included different studies than Staub et al. Three papers included in our review, but not theirs, had sensitivities of 40%,Reference Soult, Weireter and Britt42 43%,Reference Abdulrahman, Musthafa and Hakim5 and 77%.Reference Ianniello, Di Giacomo, Sessa and Miele43 The two papers not included herein but cited by the aforementioned review had sensitivities of 83%Reference Mumtaz, Zahur, Chaudhry and Warraich44 and 95%.Reference Ojaghi Haghighi, Adimi, Shams Vahdati and Sarkhoshi Khiavi45 These likely explain the sensitivity differences. However, whether having a sensitivity of 69% or 81%, ultrasound still does not perform well enough to be used as a rule-out test.
Identification of a PCE
Our pooled results suggest excellent sensitivity and specificity of eFAST in the detection of a PCE in the trauma setting with a positive likelihood ratio of 34.169 and negative likelihood ratio of 0.110 (Table 2). Two papersReference Nicol, Navsaria, Beningfield, Hommes and Kahn19,Reference Boulanger, Kearney, Tsuei and Ochoa20 presented outlying results, with one paperReference Nicol, Navsaria, Beningfield, Hommes and Kahn19 having a specificity of 5%. While not directly commented upon in the original publication, this appears to be because of including only penetrating injuries to the cardiac box, which would have a high pretest probability of injury, resulting in few true negative scans and an overall low specificity. In the other study, only one true positive result was returned,Reference Boulanger, Kearney, Tsuei and Ochoa20 limiting this study. Removal of these outlying papers from the analysis resulted in a sensitivity of 98.2% and specificity of 98.5%. These adjusted results better reflect the summary of available literature. A false positive PCE scan can result from epicardial fat or pleural fluid, but false negatives can be because of small volumes of PCE or pericardial lacerations.
Identification of free intra-abdominal fluid
Our pooled results suggest a moderate sensitivity and excellent specificity of eFAST in the detection of FF corresponding to a positive likelihood ratio of 20.3 and negative likelihood ratio of 0.25 (Table 3). These test characteristics did not change significantly if pediatric, hypotensive, and adult normotensive subgroups were considered. If considering use, it should be noted again that there is the potential for false positives (e.g., perinephric fat and abdominal ascites) and false negatives (e.g., a small volume of fluid). Lastly, as there were high degrees of heterogeneity amongst included studies, clinician judgment should predominate while considering disposition based on ultrasound imaging.
Limitations
Our study had several potential limitations. First and foremost, our study was potentially limited by our search strategy. To minimize selection bias, we employed an expert librarian to develop our search strategy and used a bibliographic review of all included studies to minimize the chances of missing important literature. Given the moderate to high heterogeneity of included studies, it is unlikely that any missed studies would have dramatically changed our final results or conclusions. Further, our results are similar to two recent reviews.Reference Staub, Biscaro, Kaszubowski and Maurici14,Reference Stengel, Bauwens and Sehouli15
We recognize the potential for publication bias, as only positive results are generally published. However, some included papers report low sensitivities and specificities, and during the history of ultrasound literature, many groups investigated whether the technology would be of benefit that is reflected by the large heterogeneity of study results. Funnel plot analysis was performed for lung and abdominal exam aspects of the eFAST and did not show evidence of publication bias (data are not shown).
Thirdly, the quality of the included studies is always a concern in systematic reviews and meta-analyses. As with study quality, we are also unable to control the statistical heterogeneity of the presented studies, by which this study is limited. If compared to the entire group of studies examining FF, the subgroup analyses had similar results, yet improved heterogeneity. While the large group was heterogeneous, these similar results in the less heterogeneous subgroups provide some reassurance to our findings.
CONCLUSIONS
Our findings suggest that eFAST can be used as a rule-in test for PTX, FF, or PCE in a trauma setting. This is supported by the high specificities and high positive likelihood ratios for each scan. Its usefulness as a rule-out tool in the trauma setting is not supported by our findings.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2019.381.








