INTRODUCTION
National surveillance indicates that most diagnosed hepatitis C virus (HCV) infections in England and Wales are in current or ex-injecting drug users (IDUs) [Reference Gungabissoon, Balogun and Ramsay1, 2]. It is therefore important to obtain accurate estimates of the incidence and prevalence of HCV infection in IDUs. A number of studies in IDUs in the United Kingdom, have found evidence of HCV infection in 30–60% of injectors [Reference Lamden3–Reference Roy5]. There are, however, no proven laboratory markers of recently acquired (incident) infection and the incidence of HCV infection is therefore difficult to determine.
Two UK-based studies, one in Glasgow using a retrospective cohort method and the other in London using a more traditional prospective approach, estimated HCV incidence rates of 28·4 and 41·8/100 person-years in IDUs respectively [Reference Roy6, Reference Judd7]. The first study used unlinked anonymous anti-HCV testing of serum residues collected from IDUs having two or more voluntary named HIV tests between 1993 and 1998. The second used a traditional prospective approach, testing oral fluids collected from IDUs in London in 2001–2002. Both studies were concentrated in areas of high HCV prevalence and included individuals who were injecting at the time of both their initial and subsequent tests. Prospective follow-up studies are both difficult and expensive and IDUs who are available for long-term follow-up may not be typical of the drug-using population as a whole.
Acute HCV infection is typically asymptomatic but when an illness occurs the average interval from exposure to symptom onset is 6–7 weeks (42–49 days) [8]. Anti-HCV antibodies can be detected in the blood of 80%, 90% and 97% of patients within 15 weeks, 5 months and 6 months, respectively, after exposure [8]. Studies of post-transfusion HCV infection have estimated that the average period before antibody is detected by third-generation HCV assays is 70 days [Reference Busch9, 10], whereas in another setting the average interval from exposure to seroconversion was 56–63 days [8]. The interval from exposure to the detection of anti-HCV is called the seroconversion window period. Before the appearance of anti-HCV antibody, however, individuals are typically viraemic, signified by the presence of HCV RNA in the blood. This is following an ‘eclipse’ phase immediately after exposure when no infectious virus is recoverable [Reference Flanagan and Barbara11], HCV RNA can often be detected in serum or plasma within 7–14 days, but occasionally may not appear until 30–40 days [8–Reference Mortimer12]. The antibody-negative, HCV RNA-positive window period has been estimated in infected donors and blood product recipients to be around 60 days [Reference Busch13].
Detection of HCV RNA-positive individuals during this anti-HCV-negative window period can therefore be used to estimate the incidence of HCV infection using individual serum specimens from cross-sectional population surveys [Reference Page-Shafer14]. In the cross-sectional study described here the prevalence of anti-HCV in IDUs who attended genitourinary medicine (GUM) clinics was estimated over time. We also employed an effective approach to identify incident infections and to estimate HCV incidence in this high-risk group.
METHODS
Anti-HCV testing
The Unlinked Anonymous Prevalence Monitoring Programme (UAPMP) began in 1990 to measure the distribution of anti-HIV-1 in accessible groups of the adult population [Reference Nicoll15]. The survey includes attenders of GUM clinics in which residues of serum specimens collected for syphilis serology are unlinked and anonymized using established methods and tested for anti-HIV-1. Specimens were stored at −20°C and as part of the GUM survey, whether participants have ever injected drugs is recorded.
To determine the prevalence of hepatitis C in specimens collected in 1998, 1999, 2000 and 2001, remaining specimens from IDUs attending 15 GUM clinics (from Wales, Northern Ireland, and the following health regions in England: East of England, London, North East, North West, West Midlands, and Yorkshire & Humberside) were tested for anti-HCV. These specimens were tested individually with the Ortho® HCV 3.0 enzyme-linked immunosorbent assay (ELISA) test system (with enhanced SAVe; Raritan, NJ, USA). Each specimen that was reactive by the Ortho assay was also tested by Monolisa® anti-HCV Plus (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). Specimens with discordant results, and those that were weakly reactive in one or both assays were further tested with a recombinant immunoblot assay (Ortho® HCV RIBA-3), and results interpreted according to the manufacturer's instructions. We previously reported a prevalence study on 1329 specimens from IDUs collected during 1995 and 1996 as part of the UAPMP GUM survey, finding an unadjusted anti-HCV prevalence of 36·9% for both years [Reference Balogun16]. Ethical clearance for the study was obtained from the ethics committee in each locality where the UAPMP operated.
Pooling and HCV RNA detection
To detect viraemic infections in the seroconversion window period (incident infections), the anti-HCV-negative specimens were tested in small pools for the presence of HCV RNA by a nested reverse transcriptase–polymerase chain reaction (RT–PCR) assay. A pool size of 10 was used as it provided significant time savings while having minimal impact on sensitivity (estimated detection limit: 120 copies/ml which, accounting for pooling, is equivalent to 1200 copies/ml in individual specimens). RNA was extracted from 100 μl of each serum pool using the Amplicor™ HCV Specimen Preparation kit (Roche Diagnostic Systems, Welwyn Garden City, Herts, UK). The RT–PCR assay utilized random priming of cDNA production and nested primer pairs which target the 5′-non-coding region (5′-NCR) of the HCV genome [Reference Harris17, Reference Li18]. If a pool was reactive, each specimen comprising the pool was tested individually for HCV RNA using the same PCR assay. Specimens that were indeterminate after Ortho® HCV RIBA-3 testing were tested individually for HCV RNA.
HCV genotyping using restriction fragment length polymorphism (RFLP) was performed on all individual RT–PCR-positive sera [Reference Harris17, Reference Poljanpelto19]. In brief, products of the PCR process were digested with each of four restriction enzymes, the digests electrophoresed and the fragment pattern analysed to derive the genotype. As a sample of anti-HCV-positive samples from 1995 and 1996 had already been genotyped [Reference Balogun16] a sample of anti-HCV-positive specimens from 2001 were tested individually for HCV RNA, and specimens found to be RT–PCR positive were genotyped to permit comparison.
Statistical analysis
Data from IDUs attending GUMs during 1995–2001 were analysed as a single group. Single variable logistic models were first fitted followed by multivariable models. Multivariable logistic regression was used to compare the prevalence of HCV by clinic, region, age, sex and sexual orientation, country of birth, and HIV serostatus. Statistical significance was taken at the 5% level. Two-way interactions between variables of interest (P<0·05) in the multivariable model were also investigated.
Confidence intervals (CI) for the prevalence of HCV RNA in the anti-HCV-negative or indeterminate specimens were calculated using the Poisson distribution. Incidence in this study encompasses window-period detection as well as HCV RNA detection in indeterminate specimens. Window-period intervals were estimated to adjust for the uncertainty around the true seroconversion time and HCV RNA-positive window period. As HCV RNA appears between 7 and 14 days following exposure and the average time to seroconversion is between 56 and 70 days [8–10], it was assumed that the average duration of the HCV RNA-positive antibody- negative window period was between 42–63 days [Reference Busch13]. Using this assumption, incidence is proportional to the prevalence of specimens falling within the window period and can therefore be estimated from cross-sectional studies [Reference Satten20, Reference Janssen21] The uncertainty range for this incidence estimate is based upon the stated HCV RNA detection times. Incidence, in this instance, denotes the number of new infections for each 100 person-years lived in the susceptible population. The Cochran–Armitage test [Reference Armitage and Colton22] was then used to test for linear trend in incidence by year.
RESULTS
Of a total of 1329 specimens from IDUs who had attended the collaborating GUM clinics in 1995 and 1996, 490 were anti-HCV seropositive, giving an unadjusted prevalence of 36·9% for both years [Reference Balogun16]. For samples from the years 1998–2001, the overall prevalence estimate was 29·3% (747/2553). Prevalence fell from 36·9% in 1995 to 28·7% in 2001 (Table 1). Anti-HCV prevalence fell during 1998–1999 and then again during 1999–2000, but rose slightly in 2001. Multivariable logistic regression analysis of these data (1995–2001) showed that anti-HCV prevalence was higher in the London area compared to the geographical area outside London (39·3% vs. 21·0%, P<0·0001) (Table 1). Results of the multivariable analysis showed that there was a marked variation in prevalence by clinic (P<0·0001), this being largely explained by the higher prevalence seen in London. Overall the majority of infections were in males (834/1237, 67·4%). There was a highly significant variation in prevalence by age (P<0·0001) with prevalence increasing with age (Table 1). In the multivariable analysis, year was significant (P=0·003) and this can be summarized as a declining trend in the overall annual prevalence (P=0·0002) where the odds ratio was 0·93 per year (95% CI 0·89–0·96).
Table 1. Multivariable analysis of IDUs who attended GUM clinics (1995–2001)
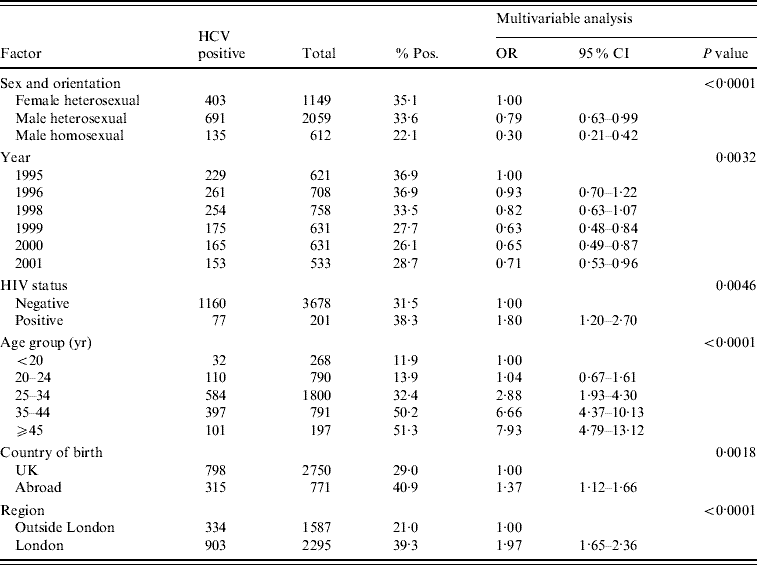
IDU, Injecting drug user; GUM, genitourinary medicine; OR, odds ratio; CI, confidence interval.
Prevalence in those born abroad was 40·9% compared to 29·0% in UK-born attendees (P=0·002). Male homosexual IDUs had a significantly lower anti-HCV prevalence (22·1%, P=0·0001) than female and male heterosexual IDUs (35·1% and 33·6%, respectively). The overall prevalence of co-infection with HIV was low, with only 6·2% of anti-HCV positives also being anti-HIV-1 positive (77/1237). There was a significantly higher prevalence of anti-HCV in the anti-HIV-1-positive group compared to the anti-HIV-1-negative group (38·3% vs. 31·5%, P=0·005). There was a significant interaction between HIV status and sexual orientation (P=0·002). Comparison of HCV prevalence within the anti-HIV-1-negative and -positive groups gave the following results: in male homosexuals, anti-HCV prevalence was similar in anti-HIV-1-positive and -negative groups (25·0% vs. 21·5% respectively, P=0·73), whereas HCV prevalence was significantly higher in anti-HIV-1-positive than anti-HIV-1-negative male (71·4% vs. 32·8%, P=0·041), and female heterosexuals (90·0 vs. 34·2%, P=0·009), respectively.
In 1995–1996, of 19 indeterminate specimens tested for HCV RNA, four were positive [Reference Balogun16]; all of the seven indeterminate specimens from later years were HCV RNA negative. A further 2506 anti-HCV-negative specimens (including 707 from 1995 and 1996) had sufficient volumes retrievable for PCR testing in pools (Table 2). Seven pools of anti-HCV-negative sera were found to contain HCV RNA and all constituent specimens were individually tested. Each of these seven reactive pools gave rise to a single RT–PCR-positive specimen. Inclusion of the four anti-HCV indeterminate/HCV RNA-positive specimens from 1995 and 1996, on the basis that these were likely to be early seroconverters, gave a total of 11 HCV RNA-positive specimens classified as incident infections (Table 3). None of these specimens were anti-HIV-1 positive.
Table 2. Specimens tested and found HCV RNA-positive by year
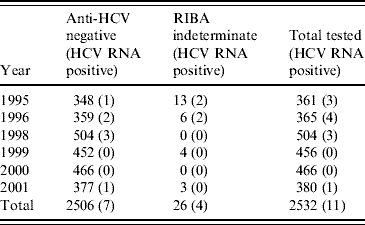
Table 3. Characteristics of IDUs on whom HCV RNA-positive/anti-HCV-negative or HCV RNA-positive/anti-HCV-indeterminate results were obtained
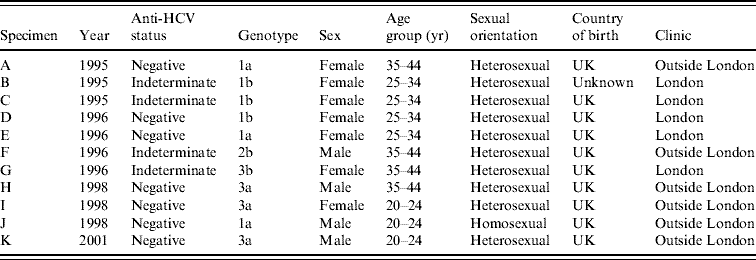
IDU, Injecting drug user.
Genotyping of the incident infections identified the most common genotype as type 1 (Table 3), with type 3 being more frequently identified after 1998. Sixty anti-HCV-positive specimens from 2001 were tested for HCV RNA for comparative purposes. Of the 60 tested, 15 (25%) contained HCV genotype 1a, three carried 1b, eight carried 3a, one carried 3b, and two carried 4d. One specimen was identified as of indeterminate genotype, six had insufficient volume for genotyping and 24 were RNA negative.
The estimated prevalence of HCV RNA in the anti-HCV antibody-negative and indeterminate specimens was 0·43% (11/2532, 95% CI 0·22–0·78). Assuming that the mean window period is between 42 and 63 days, the observed prevalence can be converted to an incidence estimate. This gives an overall point estimate of incidence of between 2·66% and 3·23% (Table 4) taking account of the uncertainty about the true window period. The annual incidence in these injectors was estimated as being 3·01% (95% CI 1·25–6·73).
Table 4. Annual incidence estimates (interval estimates) of hepatitis C in IDUs attending GUM clinics
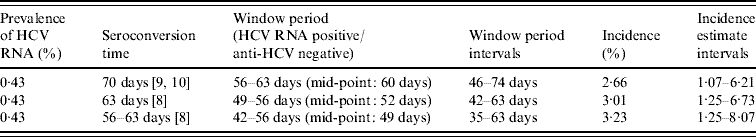
IDU, Injecting drug user; GUM, genitourinary medicine.
A significant downward trend in incidence was detected between 1996 and 2001 (P=0·009). The average annual decrease in the proportion of HCV RNA-positive specimens over the period 1995–2001 was estimated as 0·17%. This converts to an annual decrease in HCV incidence of 1·2% on the basis of a midpoint duration of pre-seroconversion viraemia of 52 days.
DISCUSSION
The prevalence and incidence of HCV in IDUs attending GUM clinics fell between 1995 and 2001. It has previously been shown that in GUM clinic attendees in England, the prevalence of HIV infection was highest in homosexual men [Reference Chadborn23]. The present study confirms the small extent of overlap between the HIV and hepatitis C epidemics in IDUs. The prevalence of HIV infection in IDUs in London was previously found to be 4·2% while that for HCV in the same study population was 43·7% [Reference Judd7].
In our study, the estimated prevalence of HCV RNA positivity in anti-HCV-negative specimens was low, at 0·43%. HCV genotyping identified that types 1a, 1b and 3a were equally dominant HCV genotypes in new infections, suggesting that different transmission chains are occurring and similar to the seroconversions identified in a Netherlands study [Reference Beld24]. The distribution of genotypes in new infections was broadly similar to that in prevalent infections.
From these incident infections, we were able to estimate an annual incidence of 3·01% for the period 1995–2001, lower than previous UK-based studies. As outlined below, the differences may be due to several factors. The only other national estimates of HCV incidence in IDUs in England and Wales were based on HCV prevalence data in those attending specialist services. This used a mathematical modelling approach and estimated that the incidence in susceptible IDUs (force of infection) over the period 1999–2003 was 16% in the first year of injecting, declining to 6% thereafter [Reference Sutton25]. Other UK studies have been conducted in areas of high hepatitis C prevalence, where HCV incidence is also expected to be high. The annual incidence was estimated to be 28·4% in Glasgow IDUs during the 1990s [Reference Roy6] and 41·8% during 2001 and 2002 in London [Reference Judd7]. In the London study, participants reported high levels of injecting risk behaviour in the previous 4 weeks. Another London study that tested stored serum from anti-HCV-negative IDUs for HCV RNA between 1999 and 2001 estimated the incidence to be 14·3% [Reference Aarons26]. Based on a low rate of RNA positivity in stored samples from anti-HCV-positive IDUs, however, the authors of the latter study suggested that the specimens may not have been stored optimally, the implication being that the true HCV incidence may have been greater [Reference Aarons26]. In our study, 29/54 (54%) specimens from anti-HCV-positive IDUs in 2001 were RNA positive, somewhat lower than the 74% expected [Reference Micallef, Kaldor and Dore27]. This suggests that our specimens may not have been stored optimally for RNA detection, and the true number of acute infections may be greater. In addition, the specimens tested were from GUM clinic attendees who had admitted ever injecting drugs and may include ex-users who had not been at recent risk of HCV infection. As the GUM clinic survey principally targets sexual risk, detailed risk information on current and past injecting history is not available. Data from the National Survey of Sexual Attitudes and Lifestyles (NATSAL) survey in 2000–2001 suggest that in those with a history of injecting who also attended GUM clinics only 21% are current injectors [28]. Using this figure, this would suggest that annual incidence in current IDUs may be around five times higher than our estimates, i.e. about 15%, more consistent with previous studies.
Another possible explanation for the low HCV incidence observed in IDUs who attended GUM clinics, would be an incorrect assumption about the duration of the anti-HCV-negative/HCV RNA-positive window period. Estimates for the window period are generally derived from studies of infected donors and blood-product recipients using third-generation ELISAs [Reference Busch13]. We assume that the natural history of HCV in injectors does not differ from those studied but if the infectious window period in our population was shorter, then the true incidence could be higher. A cohort study of 358 IDUs in The Netherlands, however, observed a prolonged period of seronegative HCV viraemia in five (all HIV negative) of 19 HCV seroconvertors [Reference Beld29]. In addition, to maximize our identification of incident infections, we included HCV RNA-positive/anti-HCV antibody-indeterminate specimens on the basis that these probably represent recently acquired HCV infections.
The wide range of HCV incidence estimates obtained in IDUs in other countries is also consistent with differing levels of risk between IDUs in different settings, at different times and recruitment methods. In Europe, annual HCV incidence was found to be 4·2% and 11·7% in IDUs on methadone maintenance programmes in Switzerland [Reference Broers30, Reference Chamot31], 26·3% in those attending syringe exchanges in Sweden [Reference Mansson32] and 9% in a prospective study in France [Reference Lucidarme33]. In the United States, annual HCV incidence rates of 6·4%, 16% and 11% were found in retrospectively identified IDUs in Baltimore [Reference Villano34], prospective studies of IDUs in Baltimore [Reference Garfein35] and young (aged <30 years) street-recruited IDUs in San Francisco, respectively [Reference Hahn36]. In Canada, a prospective study in street-recruited injectors found an incidence of 29% [Reference Patrick37] and the incidence was 27% in a retrospective study in Quebec [Reference Roy38]. Two recent cohort studies in Australia have found HCV incidence rates of around 30% [Reference Maher39, Reference Maher40].
We demonstrated that incidence in our study population declined over the period 1995–2001. Although the proportion of anti-HCV-positive samples that were HCV RNA positive was slightly higher (64%) in 1995–1996 [Reference Balogun16], storage conditions for the specimens were similar, and we do not believe that this decline could be explained by declining sensitivity of the technique. Evidence from Scotland suggests that the incidence of HCV infection declined in the early to mid 1990s [Reference Roy5], but no major change in the force of infection was detected in England and Wales over the period 1999–2003 [Reference Sutton25]. It is possible, that the decline in incidence observed in our study is due to a change in the population under study, for example, by including more ex-users in later years. The accompanying decline in prevalence is also consistent with lower risk in our study population in later years. Incidence may have increased after 2001, consistent with the higher prevalence rates in the UAPMP [41].
Further large scale cross-sectional studies are necessary to examine the validity of estimating HCV incidence through PCR approaches such as that described here in comparison with the conventional follow-up studies. The approach adopted in the present study presents an efficient means of estimating HCV incidence in unlinked anonymous surveys. Such strategies can be used effectively to inform ongoing public health surveillance. Assuming that the sensitivity of assays does not vary with time, the results obtained are valid for inferring trends in incidence in IDUs over time and between localities.
ACKNOWLEDGEMENTS
We thank all the laboratory and administrative staff who contribute to the Unlinked Anonymous Prevalence Monitoring Programme Genitourinary Medicine survey. We also thank Florian Bobet and Mark Williams for their input into the laboratory testing, Kim Lewis for coordinating aspects of the testing and Gary Murphy and Nigel Wallis for overseeing the genotyping of the prevalent infections. Funding for the study was provided by the Department of Health and the Public Health Laboratory Service Small Scientific Initiative Fund.
DECLARATION OF INTEREST
None.






