Article contents
Engineering mesoporous silica for superior optical and thermal properties
Published online by Cambridge University Press: 16 November 2020
Abstract
We report a significant advance in thermally insulating transparent materials: silica-based monoliths with controlled porosity which exhibit the transparency of windows in combination with a thermal conductivity comparable to aerogels.
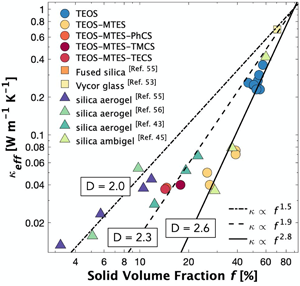
The lack of transparent, thermally insulating windows leads to substantial heat loss in commercial and residential buildings, which accounts for ~4.2% of primary US energy consumption annually. The present study provides a potential solution to this problem by demonstrating that ambiently dried silica aerogel monoliths, i.e., ambigels, can simultaneously achieve high optical transparency and low thermal conductivity without supercritical drying. A combination of tetraethoxysilane, methyltriethoxysilane, and post-gelation surface modification precursors were used to synthesize ambiently dried materials with varying pore fractions and pore sizes. By controlling the synthesis and processing conditions, 0.5–3 mm thick mesoporous monoliths with transmittance >95% and a thermal conductivity of 0.04 W/(m K) were produced. A narrow pore size distribution, <15 nm, led to the excellent transparency and low haze, while porosity in excess of 80% resulted in low thermal conductivity. A thermal transport model considering fractal dimension and phonon-boundary scattering is proposed to explain the low effective thermal conductivity measured. This work offers new insights into the design of transparent, energy saving windows.
- Type
- Original Research Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 12
- Cited by



