Published online by Cambridge University Press: 03 February 2022
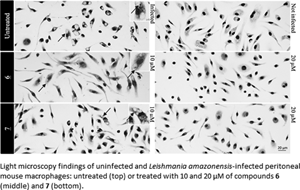
Cutaneous leishmaniasis (CL) is a spectrum of clinical manifestations characterized by severe skin ulcerations that leads to social stigma. There are limited treatment options for CL, and the available drugs are becoming less efficacious due to drug resistance. More efficacious and safer antileishmanial drugs are needed. In this study, the biological effect of seven synthetically accessible nitroaromatic compounds was evaluated in vitro against amastigotes of Leishmania amazonensis, followed by in vivo evaluation using mouse models of CL. Two compounds (6 and 7) were active against amastigotes in vitro [half-maximal effective concentration (EC50): 4.57 ± 0.08 and 9.19 ± 0.68 μm, respectively], with selectivity indexes >50, and the other compounds were not selective. In vivo, compounds 6 and 7 (10 mg kg−1, twice a day for 14 days) failed to reduce skin lesion sizes and parasite loads determined by light microscopy of lesion imprints and quantitative polymerase chain reaction. Nevertheless, the in vitro leishmanicidal efficacy sustained their use as templates for nitroimidazole-based antileishmanial drug discovery programmes focusing on analogues with more suitable properties.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.