Introduction
Bipolar disorder (BD) is a severe, recurrent mood disorder characterized by a substantial burden of depression and mania symptoms, alongside comorbid psychiatric conditions (Vieta et al., Reference Vieta, Berk, Schulze, Carvalho, Suppes, Calabrese and Grande2018). Additionally, cardiovascular disease (CVD) is excessively prevalent in BD and occurs over a decade prematurely (Goldstein, Schaffer, Wang, & Blanco, Reference Goldstein, Schaffer, Wang and Blanco2015b; Nielsen, Banner, & Jensen, Reference Nielsen, Banner and Jensen2021; Westman et al., Reference Westman, Hällgren, Wahlbeck, Erlinge, Alfredsson and Ösby2013). An American Heart Association scientific statement positioned BD as a condition that predisposes youth to accelerated atherosclerosis and early CVD (Goldstein et al., Reference Goldstein, Carnethon, Matthews, McIntyre, Miller, Raghuveer and McCrindle2015a). Importantly, the excessive rates and premature onset of CVD exceeds what can be explained by traditional cardiovascular risk factors (e.g. hypertension, obesity, smoking), psychiatric medications, and substance use (Goldstein et al., Reference Goldstein, Schaffer, Wang and Blanco2015b).
This prompts the question of what other factors may contribute to the excess and prematurity of CVD, beyond traditional cardiovascular risk factors. While ischemic heart disease, a condition excessively prevalent in BD, is most commonly caused by atherosclerosis (Hsu, Chien, & Lin, Reference Hsu, Chien and Lin2021), other processes may explain and contribute to the BD-heart connection. For example, depression (Pimple et al., Reference Pimple, Hammadah, Wilmot, Ramadan, Mheid, Levantsevych and Vaccarino2019; Wei et al., Reference Wei, Pimple, Shah, Rooks, Bremner, Nye and Vaccarino2014), stress (Pimple et al., Reference Pimple, Hammadah, Wilmot, Ramadan, Mheid, Levantsevych and Vaccarino2019), discrimination (McKinnon et al., Reference McKinnon, Shah, Lima, Moazzami, Young, Sullivan and Lewis2021), and anger (Pimple et al., Reference Pimple, Shah, Rooks, Bremner, Nye, Ibeanu and Vaccarino2015) are associated with mental stress-induced myocardial ischemia, independent of demographic factors (e.g. sex, race, socioeconomic status) (McKinnon et al., Reference McKinnon, Shah, Lima, Moazzami, Young, Sullivan and Lewis2021; Pimple et al., Reference Pimple, Shah, Rooks, Bremner, Nye, Ibeanu and Vaccarino2015; Wei et al., Reference Wei, Pimple, Shah, Rooks, Bremner, Nye and Vaccarino2014), medications (e.g. antidepressants, statins, beta-blockers) (Pimple et al., Reference Pimple, Hammadah, Wilmot, Ramadan, Mheid, Levantsevych and Vaccarino2019; Wei et al., Reference Wei, Pimple, Shah, Rooks, Bremner, Nye and Vaccarino2014), cardiovascular risk (e.g. smoking, hypertension, diabetes) (McKinnon et al., Reference McKinnon, Shah, Lima, Moazzami, Young, Sullivan and Lewis2021; Pimple et al., Reference Pimple, Shah, Rooks, Bremner, Nye, Ibeanu and Vaccarino2015; Wei et al., Reference Wei, Pimple, Shah, Rooks, Bremner, Nye and Vaccarino2014), and CVD severity (McKinnon et al., Reference McKinnon, Shah, Lima, Moazzami, Young, Sullivan and Lewis2021; Pimple et al., Reference Pimple, Shah, Rooks, Bremner, Nye, Ibeanu and Vaccarino2015; Wei et al., Reference Wei, Pimple, Shah, Rooks, Bremner, Nye and Vaccarino2014). Takotsubo cardiomyopathy, also known as broken heart syndrome, is characterized by left ventricular dysfunction after experiencing major emotional or psychological stressors (Pelliccia, Kaski, Crea, & Camici, Reference Pelliccia, Kaski, Crea and Camici2017). Both stress-induced myocardial ischemia and takotsubo cardiomyopathy are thought to be related to microvascular dysfunction, even in the absence of coronary atherosclerosis (Pelliccia et al., Reference Pelliccia, Kaski, Crea and Camici2017; Vaccarino et al., Reference Vaccarino, Shah, Mehta, Pearce, Raggi, Bremner and Quyyumi2021a; Vitale, Rosano, & Kaski, Reference Vitale, Rosano and Kaski2016). We posit that BD, in part, is subserved by microvascular dysfunction (Goldstein, Reference Goldstein2017; Goldstein et al., Reference Goldstein, Baune, Bond, Chen, Eyler, Fagiolini and Fiedorowicz2020), and that this may contribute to the increased risk of CVD, beyond traditional cardiovascular risk factors.
Myocardial flow reserve (MFR), a measure of myocardial microvascular function, refers to the vasodilatory capacity of the myocardial microvessels to increase myocardial perfusion in response to vasoactive substances such as adenosine (Kaufmann & Camici, Reference Kaufmann and Camici2005). MFR is impaired in adults with type 2 diabetes (Atar, Altuner, Bozbas, & Korkmaz, Reference Atar, Altuner, Bozbas and Korkmaz2012; Nahser, Brown, Oskarsson, Winniford, & Rossen, Reference Nahser, Brown, Oskarsson, Winniford and Rossen1995), hypertension (Galderisi et al., Reference Galderisi, de Simone, Cicala, Parisi, D'Errico, Innelli and de Divitiis2007; Kozàkovà et al., Reference Kozàkovà, Palombo, Pratali, Pittella, Galetta and L'Abbate1997; Rimoldi, Rosen, & Camici, Reference Rimoldi, Rosen and Camici2014), and hypercholesterolemia (Galderisi et al., Reference Galderisi, de Simone, Cicala, Parisi, D'Errico, Innelli and de Divitiis2007; Yokoyama et al., Reference Yokoyama, Ohtake, Momomura, Nishikawa, Sasaki and Omata1996). Additionally, psychological stressors, such as mental stress (Arrighi et al., Reference Arrighi, Burg, Cohen, Kao, Pfau, Caulin-Glaser and Soufer2000; Hasegawa et al., Reference Hasegawa, Daimon, Toyoda, Teramoto, Sekine, Kawata and Komuro2005), post-traumatic stress disorder (Vaccarino et al., Reference Vaccarino, Goldberg, Rooks, Shah, Veledar, Faber and Bremner2013; Vaccarino et al., Reference Vaccarino, Shah, Moncayo, Nye, Piccinelli, Ko and Bremner2021b), and depression (Vaccarino et al., Reference Vaccarino, Votaw, Faber, Veledar, Murrah, Jones and Bremner2009) have also been associated with impaired MFR. Furthermore, impaired MFR has also been observed in youth with Kawasaki disease (an illness characterized in part by inflammation and swelling of the coronary vessels) (Furuyama et al., Reference Furuyama, Odagawa, Katoh, Iwado, Ito, Noriyasu and Tamaki2003; Hamaoka, Onouchi, & Ohmochi, Reference Hamaoka, Onouchi and Ohmochi1992; Muzik et al., Reference Muzik, Paridon, Singh, Morrow, Dayanikli and Di Carli1996; Noto et al., Reference Noto, Karasawa, Kanamaru, Ayusawa, Sumitomo, Okada and Harada2002), and in children and neonates with congenital heart disease (Aburawi & Pesonen, Reference Aburawi and Pesonen2011; Bengel et al., Reference Bengel, Hauser, Duvernoy, Kuehn, Ziegler, Stollfuss and Hess1998). The gold standard measure of MFR is based on positron emission tomography with the injection of adenosine, which exposes individuals to radiation and arrhythmogenic risk (Li et al., Reference Li, Csepe, Hansen, Sul, Kalyanasundaram, Zakharkin and Fedorov2016; Singh & McKintosh, Reference Singh and McKintosh2021). Therefore, non-invasive approaches optimize the risk-benefit balance in certain populations, such as youth and individuals without clinical cardiovascular pathology.
Coronary microvascular reactivity (CMVR), defined as increased myocardial oxygenation in response to vasoactive stimuli (Fischer et al., Reference Fischer, Yamaji, Luescher, Ueki, Jung, von Tengg-Kobligk and Guensch2018; Fischer, Guensch, & Friedrich, Reference Fischer, Guensch and Friedrich2015), is a non-invasive measure of coronary microvascular function. CMVR can be measured via oxygen-sensitive cardiovascular magnetic resonance imaging (OS-CMR) using a combined breathing-paradigm of hyperventilation to elicit hypocapnia, followed by breath-holding to elicit hypercapnia (Fischer et al., Reference Fischer, Guensch and Friedrich2015; Guensch et al., Reference Guensch, Fischer, Flewitt, Yu, Lukic, Friedrich and Friedrich2014; Teixeira, Nadeshalingam, Fischer, Marcotte, & Friedrich, Reference Teixeira, Nadeshalingam, Fischer, Marcotte and Friedrich2016). In addition to the advantages of non-invasiveness and a strong safety and tolerability profile, CMVR exhibits sensitivity across the full physiological spectrum of myocardial microvascular function (Fischer et al., Reference Fischer, Guensch and Friedrich2015). This sensitivity is attributed to the fact that breathing-paradigms assess the physiological mechanism of preserving myocardial oxygenation, which is regulated by the arterial partial pressure of oxygen and carbon dioxide (Broten & Feigl, Reference Broten and Feigl1992; Broten, Romson, Fullerton, Van Winkle, & Feigl, Reference Broten, Romson, Fullerton, Van Winkle and Feigl1991).
We set out to examine CMVR in youth with BD v. controls. Our primary hypothesis was that youth with BD would have a lower CMVR compared to controls. Additionally, we evaluated gross cardiac structure (e.g. left ventricular mass) and/or function (e.g. ejection fraction), using MRI, although we did not expect between-group differences.
Methods
Participant recruitment for this study began in January 2017 and concluded in February 2020 due to the onset of the pandemic, followed by our research group's relocation to a different institution with a different MRI scanner and sequence. Consent was obtained from all participants and their parent and/or guardian prior to participating. Ethical approval was granted by the Research Ethics Boards at Sunnybrook Research Institute (REB 435-2015) and at the Centre for Addiction and Mental Health (REB 163/2020).
Participants
This study included youth between the ages of 13–20 years with BD (type I, II or not otherwise specified [NOS]) and controls. BD participants were primarily recruited from a tertiary subspecialty youth BD clinic. Control participants were recruited through public advertisements.
Psychiatric diagnoses of all participants were determined via the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime version (KSADS-PL) (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997). The Diagnostic and Statistical Manual of Mental Disorders IV was used to define BD type I and II. BD-NOS diagnosis was defined using the same operationalized criteria as the Course and Outcome of Bipolar Youth Study (Birmaher et al., Reference Birmaher, Axelson, Goldstein, Strober, Gill, Hunt and Keller2009). This study was recruited from an ongoing larger study from our clinic that began in 2012. Given that DSM V was released in 2013, and it took until 2016 for the updated KSADS to be released, we continued using the DSM IV criteria for consistency across the studies. The depression and mania rating scales from K-SADS-PL were used to assess current and lifetime symptom severity (Axelson et al., Reference Axelson, Birmaher, Brent, Wassick, Hoover, Bridge and Ryan2003). The K-SADS-PL post-traumatic stress disorder screener was used to obtain lifetime physical and sexual abuse history. The Children's Global Assessment Scale (CGAS) was used as a global assessment of psycho-social functioning and current and lifetime psychiatric symptoms (Shaffer et al., Reference Shaffer, Gould, Brasic, Ambrosini, Fisher, Bird and Aluwahlia1983). The socioeconomic status of parents was assessed using the Hollingshead Four-Factor Index (Hollingshed, Reference Hollingshead1975). The ethnicity and sex of participants were recorded based on participant self-reports. All interviewers had a bachelor's or master's degrees and completed training under the supervision of the senior author (B.I.G.). Diagnoses and symptom ratings were reviewed with a licensed child-adolescent psychiatrist (B.I.G.). The mood of participants with BD were classified as euthymic, depressed, and/or hypomanic/manic based on a score of: <13 on both the K-SADS-PL depression and mania scales, ⩾13 on the K-SADS-PL depression rating scale and ⩾13 on the K-SADS-PL mania rating scale respectively.
Control participants were excluded if they had a lifetime history of MDD, BD, or psychosis, or a first or second-degree family history of BD or psychosis. Participants in both groups were excluded if they were unable to provide informed consent; had substance dependence in the past three months; were taking any anti-inflammatory, anti-platelet, anti-lipidemic, anti-hypertensive, or hypoglycemic agents; or had a cardiac condition, MRI contraindications such as claustrophobia, or an infectious illness in the past 14 days.
Anthropometric measures
Height and weight measurements were obtained using standardized procedures (Krebs et al., Reference Krebs, Himes, Jacobson, Nicklas, Guilday and Styne2007) and repeated twice for accuracy. To adjust for clothing differences weight was adjusted as follows: −1.3 kg if the participant was wearing long-pants and a long-sleeved shirt, −1.1 kg for short-pants or short-sleeves, and −0.9 kg for short-sleeves and short-pants.
Image acquisition
MRI data was collected with a 3 T Siemens Prisma scanner (Siemens Healthineers, Erlangen, Germany) using a 32-channel cardiac phased array coil placed on the chest, at the level of the heart. Cardiac imaging consisted of stacks of 12–15 slices of long-axis and short-axis cardiac-gated, true fast imaging with steady-state free precession (TRUFI) sequences showing dynamic heart function (also known as cine steady-state free precession (SSFP) sequences). Two, three, and four chamber views and a short axis stack were used to evaluate left ventricular (LV) structure and function (i.e. end-diastolic volume [EDV], end-systolic volume [ESV], systolic volume [SV], mass during diastole, and ejection fraction [EF]). Typical imaging parameters were: repetition time (TR) 3.51 ms, echo time (TE) 1.54 ms, spatial resolution 1.3 × 1.3 × 8.0 mm, field of view 270 × 320 mm, matrix 216 × 256, flip angle 49°. T1 mapping was performed using a modified Look-Locker (MOLLI) inversion recovery TRUFI sequence (Messroghli et al., Reference Messroghli, Radjenovic, Kozerke, Higgins, Sivananthan and Ridgway2004) with a 5-3-3 pattern at a mid-ventricular slice with the following parameters: TR 3.89 ms, TE 1.12 ms, TI 180 ms, spatial resolution 1.4 × 1.4 × 8.0 mm, field of view 306 × 360 mm, matrix 254 × 218, flip angle 35°. T2 mapping at a mid-ventricular slice was performed using a T 2-prepared fast low angle shot (FLASH) sequence (Wright, Hu, & Macovski, Reference Wright, Hu and Macovski1991) with the following parameters: TR 3.81 ms, TE (0, 30, 55 ms), spatial resolution 1.6 × 1.6 × 8.0 mm, field of view 289 × 359 mm, matrix 224 × 180, flip angle 12°. Motion-corrected pixel-wise T 2 maps were automatically generated by the scanner.
Breathing-paradigm
Figure 1 depicts the CMVR imaging protocol, which utilized an established and validated breathing-paradigm (Fischer et al., Reference Fischer, Guensch and Friedrich2015; Guensch et al., Reference Guensch, Fischer, Flewitt, Yu, Lukic, Friedrich and Friedrich2014; Teixeira et al., Reference Teixeira, Nadeshalingam, Fischer, Marcotte and Friedrich2016). First a baseline quantitative T 2 scan was acquired. Then participants were asked to hyperventilate for 60-s at 32 breaths per minute, inhaling between metronome beats and exhaling during metronome beats. Afterwards, participants were told to exhale fully, and subsequently perform a 40-s breath-hold, during which four additional quantitative T 2 scans were acquired at 10-, 20-, 30-, and 40-s post hyperventilation. Prior to entering the MRI, the protocol was explained to all participants, and they were asked to practice the breathing-paradigm with a trained staff member until they were able to adhere to the task. Adherence to the task during the MRI scan was monitored via respiratory bellows by a trained staff member.
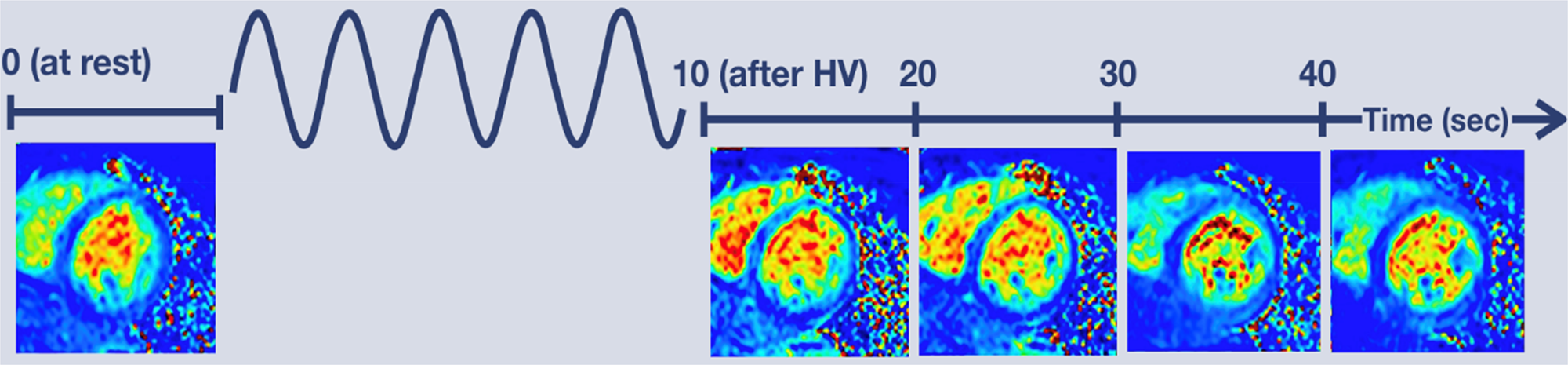
Figure 1. CMVR breathing paradigm and imaging protocol. A baseline quantitative T2 scan was acquired then participants performed a 60-s hyperventilation task with a respiration rate of 32 breaths per minute. Afterwards participants fully exhaled and then performed a 40 s breath-hold during which four additional quantitative T2 scans were acquired at 10-, 20-, 30-, and 40-s post hyperventilation.
Image processing and analysis
The two study cardiologists were masked to participant diagnosis by excluding any identifiable participant information within the MRI system. Non-imaging clinical and psychiatric data were kept in password-protected datasets that were not accessible by the cardiologists. Finally, the overall statistical analyses were conducted using a masked dummy group variable. All cardiac images were analyzed by a Society for Cardiovascular Magnetic Resonance Level 3 expert cardiologist using cvi42 (Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). The 12-15 short- and long-axis cardiac-gated cine images of the LV were analyzed slice by slice by manually contouring the endocardial and epicardial borders, yielding the following structural and functional measures of the LV for: EDV, ESV, SV, mass during diastole and EF. Similarly, the endocardial and epicardial borders were manually contoured in the T 2 scans across each time point to yield CMVR.
Statistical analysis
Clinical study data were collected and managed using REDCap (Research Electronic Data Capture) (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009). The normality of all continuous variables was assessed using the Shapiro–Wilks test. Between-group differences were examined using independent-samples t tests or Mann–Whitney U tests for continuous variables and χ2 tests for categorical variables. Effect sizes were reported as a Cohen's d for continuous variables and Cramer's V for categorical variables. Statistical testing of clinical and demographics were performed using SPSS software version 26 (IBM Inc.). p values < 0.05 were considered statistically significant.
Statistical testing of clinical measures of cardiac structure and function, and CMVR was conducted in Matlab R2021b. General linear models investigated whether clinical measures of cardiac structure or function differed between youth with BD v. controls. In these models, clinical measures of cardiac structure or function (e.g. LV-EDV) were the dependent variables; diagnosis and sex were categorical fixed variables, and age was a continuous fixed variable. Linear mixed effects models examined CMVR (i.e. change in T 2 relaxation time during the breathing-paradigm) in youth and whether CMVR differed between youth with BD v. controls. In these models, T 2 relaxation time was the dependent variable; diagnosis and sex were categorical fixed effects and age, time, body-mass-index (BMI), and time × diagnosis were continuous fixed effects; time and participant were random effects which allowed for the intercept to vary by time and subject, and for a correlated random slope of time. Post-hoc analyses at each time point were conducted to determine where CMVR in youth with BD diverged from control youth. To correct for multiple post-hoc analyses the Benjamini, Krieger, and Yekutieli two-stage step-up method of controlling for false discovery rate (FDR) was applied (Benjamini, Krieger, & Yekutieli, Reference Benjamini, Krieger and Yekutieli2006). Additionally, linear mixed-effects models examined CMVR within each group separately using the aforementioned models without the fixed effects of diagnosis and time × diagnosis. Post-hoc pair-wise comparisons examined for when a significant change in CMVR relative to baseline occurred. Lastly, sensitivity analyses controlled for the potential confounding effects of medication usage, race, and duration of illness by running separate linear mixed effects models with the addition of either current use of second-generation antipsychotics, lithium, stimulants, any medication use, or race as categorical fixed effects or duration of illness and as a continuous fixed effect.
Results
This study enrolled 92 participants, of which 6 (3 controls, 3 BD) were withdrawn from the study either because a substance use disorder was identified after enrollment (1 control, 1 BD) or the participant did not complete cardiac MRI (2 controls, 2 BD). This study included 86 youth, 39 with BD (19 BD-I, 8 BD-II, 12 BD-NOS) and 47 controls. Within the BD group, 17 (44%) participants were euthymic, 18 (46%) had depression, and 9 (23%) had hypomania. Analyses for LV parameters included a total of 84 participants, as data for two BD-I participants were not obtained for technical reasons. Analyses for CMVR included a total of 83 participants, as one BD-I participant and two control participants had excessive motion during the scan. The BD group compared to the control group, had a significantly higher proportion of Caucasian participants (72% v. 50%; p = 0.04; V = 0.22), female participants (69% v. 45%; p = 0.02; V = 0.25), and higher BMI (23.28 ± 3.42 v. 21.29 ± 2.84; p = 0.004; d = 0.64). Psychiatric clinical characteristics are summarized in Table 1.
Table 1. Demographic and clinical characteristics of participants

ADHD, attention deficit/hyperactivity disorder; ODD, oppositional defiant disorder; CD, conduct disorder; SUD, substance use disorder; SSRI, selective serotonin reuptake inhibitor; CGAS, Children's Global Assessment Scale; s.d., standard deviation.
Left ventricular parameters in youth
The between-group differences (i.e. BD v. controls) in clinical measures of left ventricular structure and function are presented in Table 2. Overall, cardiac structure and function metrics were in the normal range in both groups, and there were no significant between-group differences in these metrics (all p > 0.05). Similarly, T 1 relaxation and baseline T 2 relaxation times were not significantly different between groups (both p > 0.05). For descriptive purposes, the clinical measures of LV structure and function were compared between males and females. Females had significantly smaller LV end-diastolic volume, end-systolic volume, systolic volume, and mass during diastole even after indexing for body surface area (all p < 0.001). Additionally, females had significantly higher T 1 relaxation (p = 0.007) and baseline T 2 relaxation times (p < 0.001) than males.
Table 2. Left ventricular parameters in youth

LV, left ventricle; EDV, end-diastolic volume; ESV, end-systolic volume; SV, systolic volume; LV-EF, left ventricle ejection fraction; a, analyses with age and sex as covariates; b, analyses with age as a covariate.
Coronary microvascular reactivity
Across all participants, the breathing-paradigm induced a significant change in T 2 relaxation time across all timepoints (i.e. CMVR; β = 0.36, p < 0.001). Post-hoc tests compared to baseline found that T 2 relaxation time was significantly lower 10 s post-hyperventilation (d = 2.3, p FDR < 0.001), and higher 20-, 30-, and 40-s post-hyperventilation (d = 1.7, d = 2.02, d = 1.93 respectively; all p FDR < 0.001). There was also a significant diagnosis-by-time interaction effect (β = −0.11, p = 0.002) on T 2 relaxation time, whereby youth with BD had lower CMVR than the control group, as shown in Fig. 2. Post-hoc tests revealed that at 20 s (d = 0.48, p FDR = 0.01), 30 s (d = 0.72, p FDR < 0.001), and 40 s (d = 0.91, p FDR < 0.001) post-hyperventilation youth with BD had a significantly lower T 2 relaxation time v. controls.
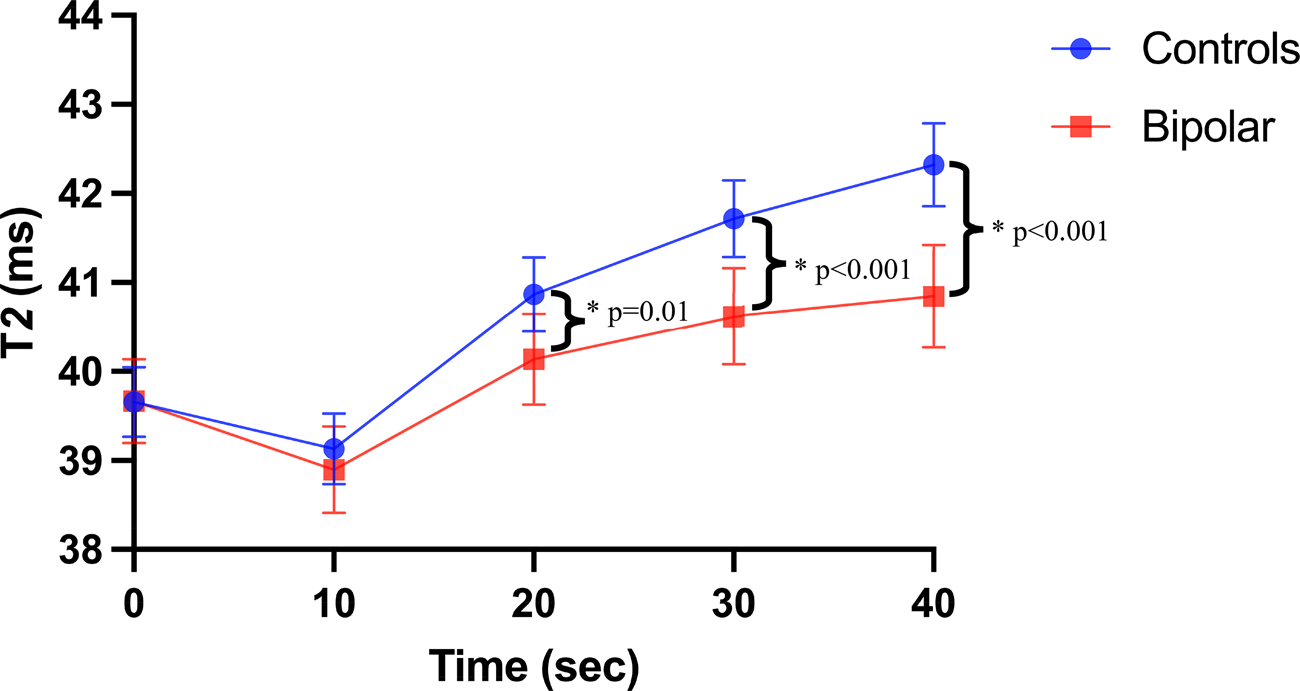
Figure 2. CMVR in youth with BD and HC. Visual representation of T2 relaxation time from quantitative T2 scans at baseline, and 10-, 20-, 30-, and 40-s post hyperventilation in youth with bipolar disorders and controls controlling for age, sex, and BMI. Youth with bipolar disorder had significantly lower CMVR than controls (β = −0.11, p = 0.002). Post-hoc tests revealed that at 20 s (d = 0.48, p FDR = 0.01), 30 s (d = 0.72, p FDR < 0.001), and 40 s (d = 0.91, p FDR < 0.001) post-hyperventilation youth with bipolar disorder had a significantly lower T2 relaxation time v. controls. Error bars = 95% confidence intervals.
Within-group analyses found that the breathing-paradigm induced a significant change in T 2 relaxation time in both the control group (β = 0.46, p < 0.001) and the BD group (β = 0.25, p = 0.002). In the control group, post-hoc tests compared to baseline found that T 2 relaxation time was lower 10 s post-hyperventilation (d = 2.3, p FDR < 0.001), and higher 20-, 30-, and 40-s post-hyperventilation (d = 3.25, d = 3.7, d = 3.6 respectively; all p FDR < 0.001). In the BD group post-hoc tests compared to baseline, found that T 2 relaxation time was lower 10 s post-hyperventilation (d = 4.58, p FDR < 0.001), and higher 20-, 30-, and 40-s post-hyperventilation (d = 1.35, d = 1.83, d = 1.69 respectively; all p FDR < 0.001). Finally, for descriptive purposes, we also compared the effect that the breathing-paradigm had on T 2 relaxation time in males v. females. However, there were no significant differences in CMVR in males v. females (β = −0.01, p = 0.75).
Sensitivity analyses
We undertook four sensitivity analyses focused on differences of medication usage in BD v. controls, specifically the current use of second-generation antipsychotics, lithium, stimulants, and any medication use. Additionally, we also conducted sensitivity analyses to control for racial differences and duration of illness in BD. All analyses remained significant after controlling for these confounds.
Discussion
This study examined an established measure of coronary microvascular function (i.e. CMVR), alongside clinical metrics of gross cardiac structure and function, in relation to BD in youth. Clinical CMR metrics of gross LV structure and function were normal in both youth with BD and controls, and did not differ between the groups. Additionally, the breathing-paradigm successfully assessed CMVR in a sample of youth without CVD, which is noteworthy as this paradigm does not require the administration of an exogenous pharmacological agent. As hypothesized, this study found evidence of coronary microvascular dysfunction in youth with BD. Importantly, this finding occurred in the context of otherwise normal cardiac structure and function and appears to be independent of BMI or psychotropic medication, including SGA. These findings provide preliminary evidence that coronary microvascular dysfunction is present in youth with BD, even in the early stages of their course of illness, and prior to any detectable abnormalities and/or differences in gross cardiac structure and function.
Coronary microvascular reactivity
Consistent with our hypothesis, we found that youth with BD had blunted CMVR compared to the control youth. These results align with prior research that found that psychological stressors (Arrighi et al., Reference Arrighi, Burg, Cohen, Kao, Pfau, Caulin-Glaser and Soufer2000; Hasegawa et al., Reference Hasegawa, Daimon, Toyoda, Teramoto, Sekine, Kawata and Komuro2005) and psychiatric disorders (e.g. major depressive disorder (Vaccarino et al., Reference Vaccarino, Votaw, Faber, Veledar, Murrah, Jones and Bremner2009), post-traumatic stress disorder (Vaccarino et al., Reference Vaccarino, Goldberg, Rooks, Shah, Veledar, Faber and Bremner2013, Reference Vaccarino, Shah, Moncayo, Nye, Piccinelli, Ko and Bremner2021b)) are associated with impaired coronary microvascular function. While impaired coronary microvascular function has been observed in children and adolescents with Kawasaki disease (Furuyama et al., Reference Furuyama, Odagawa, Katoh, Iwado, Ito, Noriyasu and Tamaki2003; Hamaoka et al., Reference Hamaoka, Onouchi and Ohmochi1992; Muzik et al., Reference Muzik, Paridon, Singh, Morrow, Dayanikli and Di Carli1996; Noto et al., Reference Noto, Karasawa, Kanamaru, Ayusawa, Sumitomo, Okada and Harada2002), and congenital heart disease (Aburawi & Pesonen, Reference Aburawi and Pesonen2011; Bengel et al., Reference Bengel, Hauser, Duvernoy, Kuehn, Ziegler, Stollfuss and Hess1998), this topic has not been previously studied in a youth psychiatric sample.
The mechanisms underlying microvascular dysfunction are multifactorial (Choi & Kim, Reference Choi and Kim2021). While the current study was not designed to elucidate mechanisms, we speculate that biological processes known to be relevant to both BD and CVD may be contributory. For example, inflammation, which is elevated in youth with BD (Karthikeyan et al., Reference Karthikeyan, Dimick, Fiksenbaum, Jeong, Birmaher, Kennedy and Goldstein2022), is independently associated with coronary microvascular dysfunction in adults with angina who do not have coronary artery disease or other cardiovascular risk factors (Recio-Mayoral, Rimoldi, Camici, & Kaski, Reference Recio-Mayoral, Rimoldi, Camici and Kaski2013). Similarly, oxidative stress is evident in youth with BD (de Sousa et al., Reference de Sousa, Zarate, Zanetti, Costa, Talib, Gattaz and Machado-Vieira2014), and is associated with microvascular dysfunction in young, otherwise healthy, adults with major depressive disorder (Greaney, Saunders, Santhanam, & Alexander, Reference Greaney, Saunders, Santhanam and Alexander2019). While compelling evidence supports that endothelial dysfunction is mediated by inflammation and oxidative stress (Cai & Harrison, Reference Cai and Harrison2000; Clapp et al., Reference Clapp, Hirschfield, Storry, Gallimore, Stidwill, Singer and Hingorani2005), other mechanisms relevant to both BD and endothelial function, such as brain-derived neurotrophic factor (Donovan et al., Reference Donovan, Lin, Wiegn, Ringstedt, Kraemer, Hahn and Hempstead2000; Goldstein et al., Reference Goldstein, Baune, Bond, Chen, Eyler, Fagiolini and Fiedorowicz2020; Totoson, Pedard, Marie, & Demougeot, Reference Totoson, Pedard, Marie and Demougeot2018), and vascular endothelial growth factor (Goldstein et al., Reference Goldstein, Baune, Bond, Chen, Eyler, Fagiolini and Fiedorowicz2020; Kliche & Waltenberger, Reference Kliche and Waltenberger2001) may have played a role in the observed findings.
The present findings build upon prior evidence from adult studies demonstrating that coronary microvascular function can be assayed non-invasively using OS-CMR with a breathing-paradigm, extending similar findings to a sample of youth without CVD. In this age group, the non-invasiveness of a breathing-paradigm relative to the administration of an exogenous pharmacological agent such as adenosine is beneficial in a research setting. Furthermore, the present findings provide evidence that OS-CMR is sensitive to detecting differences in CMVR in an otherwise healthy sample of youth with BD who are at an elevated risk of developing future CVD.
Left ventricular parameters in youth
In contrast to prior echocardiography and CMR studies in adults, which have found that depression, schizophrenia, and BD are associated with poorer measures of LV function and structure (Andreou et al., Reference Andreou, Saetre, Fors, Nilsson, Kullberg, Jönsson and Agartz2020; Chen et al., Reference Chen, Chiang, Hsiao, Shen, Lin, Chung and Tsai2021; Chen & Chung, Reference Chen and Chung2018; Kim et al., Reference Kim, Kim, Lim, Cho, Baik, Lim and Shin2012; Korkmaz, Korkmaz, Özer, & Atmaca, Reference Korkmaz, Korkmaz, Özer and Atmaca2016; Pillinger et al., Reference Pillinger, Osimo, de Marvao, Berry, Whitehurst, Statton and Howes2019), there were no significant differences in LV volumes or mass between youth with BD and controls. However, this could be expected, given that this is an otherwise healthy youth sample with a relatively short duration of illness. While BD is associated with a premature onset of CVD and predisposes youth to accelerated atherosclerosis (Goldstein et al., Reference Goldstein, Carnethon, Matthews, McIntyre, Miller, Raghuveer and McCrindle2015a, Reference Goldstein, Schaffer, Wang and Blanco2015b), the onset of CVD still does not usually occur until adulthood (Goldstein et al., Reference Goldstein, Schaffer, Wang and Blanco2015b). It is likely that changes in clinical measures of cardiac structure and function take decades to manifest and are not yet evident during youth. Therefore, it is important to investigate novel indicators of early coronary microvascular dysfunction that may precede changes in gross cardiac structure and function.
Aside from the focus on BD, the current study addresses an important gap in the knowledge regarding cardiovascular MRI in youth, a topic that focuses thus far, almost exclusively on congenital heart disease and Kawasaki disease. This is unfortunate, as normative modeling of CMR in populations of youth at an increased risk for CVD, and amongst youth in general, offers potential insights regarding the genesis and early precursors of CVD. The current study found that measures of gross cardiac structure and function were normal in both the youth with BD and controls. Additionally, males also had larger LV volumes and mass, even after indexing for body-surface area, when compared to females. This aligns with both youth and adult literature, which has shown that males have up to 40% larger LV volumes and mass (Kawel-Boehm et al., Reference Kawel-Boehm, Maceira, Valsangiacomo-Buechel, Vogel-Claussen, Turkbey, Williams and Bluemke2015; Salton et al., Reference Salton, Chuang, O'Donnell, Kupka, Larson, Kissinger and Manning2002; Sandstede et al., Reference Sandstede, Lipke, Beer, Hofmann, Pabst, Kenn and Hahn2000; van der Ven et al., Reference van der Ven, Sadighy, Valsangiacomo Buechel, Sarikouch, Robbers-Visser, Kellenberger and Helbing2020). Importantly, our CMR measures of clinical cardiac structure and function showed good agreement with reference CMR values reported in a recent multicenter study based on youth between the ages of 12–18 (van der Ven et al., Reference van der Ven, Sadighy, Valsangiacomo Buechel, Sarikouch, Robbers-Visser, Kellenberger and Helbing2020). Lastly, our study also found that females had significantly higher T 1 and T 2 relaxation times than males, which aligns with the prior adult literature (Bönner et al., Reference Bönner, Janzarik, Jacoby, Spieker, Schnackenburg, Range and Kelm2015; Granitz et al., Reference Granitz, Motloch, Granitz, Meissnitzer, Hitzl, Hergan and Schlattau2019; Piechnik et al., Reference Piechnik, Ferreira, Lewandowski, Ntusi, Banerjee, Holloway and Robson2013; Rauhalammi et al., Reference Rauhalammi, Mangion, Barrientos, Carrick, Clerfond, McClure and Berry2016).
Potential clinical applications and generalizability of findings
Generating insights and knowledge regarding the genesis and early precursors of CVD in youth, particularly among those at increased risk for CVD, is essential to inform future clinical practices. The rates of premature and excessive CVD observed in BD exceed what can be explained by traditional cardiovascular risk factors (Goldstein et al., Reference Goldstein, Schaffer, Wang and Blanco2015b), which provides motivation for a focus on microvessels. By demonstrating coronary microvascular dysfunction in youth early in their course of BD, independent of obesity and medications, the current study adds important preliminary evidence that microvessels may be implicated in the excess and premature onset of CVD in BD. There is a small but growing body of literature showing that microvascular dysfunction is present in BD and has psychiatric clinical correlates (Fiedorowicz, Coryell, Rice, Warren, & Haynes, Reference Fiedorowicz, Coryell, Rice, Warren and Haynes2012; Kennedy et al., Reference Kennedy, Islam, Karthikeyan, Metcalfe, McCrindle, MacIntosh and Goldstein2023a, Reference Kennedy, Karthikeyan, Sultan, McCrindle, Miller and Goldstein2023b; Mio et al., Reference Mio, Kennedy, Dimick, Sultan, Fiksenbaum, Selkirk and Goldstein2021; Urback, Metcalfe, Korczak, MacIntosh, & Goldstein, Reference Urback, Metcalfe, Korczak, MacIntosh and Goldstein2019). While BD is listed as a tier II moderate-risk condition for future CVD by the American Heart Association (Goldstein et al., Reference Goldstein, Carnethon, Matthews, McIntyre, Miller, Raghuveer and McCrindle2015a), current guidelines for screening and intervention focus solely on traditional cardiovascular risk factors (Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, 2011; de Ferranti et al., Reference de Ferranti, Steinberger, Ameduri, Baker, Gooding and Kelly2019). As a result, microvascular dysfunction has not yet been integrated into the appraisal of CVD risk and related screening and prevention approaches.
While additional research is needed to change future clinical practices, it is possible that, in the future, microvascular metrics may guide prevention and treatment strategies, and inform more personalized CVD risk assessment among individuals with BD and other illnesses characterized by microvascular dysfunction. For example, thresholds for CVD-related intervention may be reduced, given the knowledge that both microvascular and macrovascular risk factors contribute to the excess risk of CVD among individuals with BD. Another implication is the potential for repurposing of microvascular-focused medications focused on improving microvascular and endothelial function. Phosphodiesterase inhibitors (e.g. sildenafil), and nitric oxide synthase inhibitors (e.g. nitroarginine, aminoguanidine) target the microvascular endothelium, leading to vasodilation (Cheitlin et al., Reference Cheitlin, Hutter, Brindis, Ganz, Kaul, Russell and Wolk1999; Melikian, Seddon, Casadei, Chowienczyk, & Shah, Reference Melikian, Seddon, Casadei, Chowienczyk and Shah2009). Given that both clinical and animal studies have demonstrated that sildenafil (Duarte-Silva et al., Reference Duarte-Silva, Filho, Barichello, Quevedo, Macedo and Peixoto2020) and nitric oxide synthase inhibitors (Wegener & Volke, Reference Wegener and Volke2010) have anti-depressant effects, the benefit-to-risk ratio of using these drugs in individuals with mood disorders may be enhanced.
It is essential to discuss the generalizability of this study's findings to other populations of youth with BD. The clinical characteristics of the current sample, including psychosis, suicidality, physical/sexual abuse, treatment, comorbid psychiatric diagnoses, and family psychiatric history, closely resembled those reported in the COBY study (Birmaher et al., Reference Birmaher, Axelson, Strober, Gill, Valeri, Chiappetta and Keller2006; Birmaher et al., Reference Birmaher, Axelson, Goldstein, Strober, Gill, Hunt and Keller2009; Esposito-Smythers et al., Reference Esposito-Smythers, Goldstein, Birmaher, Goldstein, Hunt, Ryan and Keller2010). Moreover, adolescents with BD in the Canadian Community Health Survey (CCHS), the most directly comparable community-based epidemiological study, also exhibit high rates of psychiatric comorbidity and suicidality (Kozloff et al., Reference Kozloff, Cheung, Schaffer, Cairney, Dewa, Veldhuizen and Levitt2010). However, it is important to note that while our clinical sample consisted of treatment-seeking individuals, less than half of the adolescents with BD in the CCHS had accessed mental health services. Finally, the lifetime prevalence of ADHD and anxiety within our control group aligns with the prevalence reported in large national surveys and epidemiological studies in both the United States and Canada (Bitsko et al., Reference Bitsko, Claussen, Lichstein, Black, Jones, Danielson and Meyer2022; Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010; Wiens et al., Reference Wiens, Bhattarai, Pedram, Dores, Williams, Bulloch and Patten2020). Taken together, the similarity of our study's clinical characteristics to those of large and epidemiological studies indicates that our findings would likely translate to the general population.
Limitations
Several limitations of this study require consideration. First, the cross-sectional design of the study precludes inferences regarding the temporal relationship between BD and CMVR. Future studies with repeated measures are necessary to inform our understanding of the life-course relationships of CMVR in youth in general and specifically in BD. Second, the efficacy and reliability of the breathing-paradigm is subject to participant compliance. Even though participants practiced with a trained staff member and their compliance was monitored, there may be unexplained variance related to pulmonary function, and/or depth of inhalation and exhalation during the breathing-paradigm. Relatedly, a thorough assessment of respiratory diseases, such as asthma, was not included in the study. The efficacy and reliability of the breathing-paradigm may be lower in the presence of such conditions. Third, CMVR was assessed on a single midventricular slice to achieve the temporal and spatial resolutions required for monitoring changes in T2 relaxation. This local measurement may not be reflective of CMVR across the entire LV. Finally, while we investigated the potential confounding effects of select covariates in this proof-of-principle study (age, sex, obesity, second-generation antipsychotics, stimulants, lithium, any medication, race), we cannot rule out the possibility of residual confounding and effects related to other factors (e.g. psychiatric comorbidity, lifestyle, early adversity).
Conclusion
We present evidence that microvascular function is impaired among youth with BD early in their course of illness and precedes changes in the clinical metrics of cardiac structure and function. These findings converge with prior findings in adults with major depressive disorder and post-traumatic stress disorder. Heuristically, studying a youth sample is beneficial, as it can inform our understanding of the role of microvascular dysfunction in the genesis of BD, offering the advantage that youth have not been exposed to decades of BD symptoms, medications, and other cardiovascular risk factors that are often comorbid. While the current study focused on BD, the approach undertaken here can be similarly applied to studying youth with other conditions associated with an increased risk of CVD and accelerated atherosclerosis, such as juvenile inflammatory arthritis, diabetes, and inflammatory bowel disease (de Ferranti et al., Reference de Ferranti, Steinberger, Ameduri, Baker, Gooding and Kelly2019). Future studies integrating larger samples, prospective follow-up, and blood-based biomarkers are warranted.
Acknowledgements
We would like to acknowledge the contribution of the staff at the Centre for Youth Bipolar Disorder, the MRI technologists Ruby Endre and Garry Detzler, and thank the youth and their families for their participation.
Funding statement
This study was funded by the Canadian Institutes of Health Research, (Grant number: PJT 162110), and University of Toronto Miner's Lamp award. Dr Goldstein also acknowledges his position as RBC Investments Chair in Children's Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University Chair between the University of Toronto, CAMH, and the CAMH Foundation. The funding sources were not involved in study design and the conduct of the research. Additionally, all authors report no conflicts of interest related to the research.







