Introduction
The global incidence of infertility is rising around the world, and half is caused by male factors, including azoospermia, which accounts for 15% of male infertility (Cocuzza et al., Reference Cocuzza, Alvarenga and Pagani2013). Azoospermia can be classified as obstructive azoospermia (OA) and non-obstructive azoospermia (NOA) based on the aetiology (Flannigan et al., Reference Flannigan, Bach and Schlegel2017; Wu et al., Reference Wu, Lin, Sun and Cheng2021). NOA mainly refers to the impairment of spermatogenesis caused by damage to testicular structure or function. NOA has typical clinical symptoms, such as testicular volume less than 15 ml and increased follicle-stimulating hormone (FSH) value (Tiseo et al., Reference Tiseo, Hayden and Tanrikut2015). To date, except for patients with low gonadotropin, there is still no ideal drug that can effectively improve the fertility of NOA patients. To achieve an effective clinical treatment outcome for NOA patients, Devroey et al. (Reference Devroey, Liu, Nagy, Goossens, Tournaye, Camus, Van Steirteghem and Silber1995) first applied intracytoplasmic sperm injection (ICSI) technology in 1995 to treat NOA patients and succeeded in pregnancy. With the widespread application of ICSI technology to infertility treatment for patients with azoospermia, to obtain an appropriate amount of sperm on the day of ovum pick-up (OPU), the method of surgical sperm retrieval has been greatly developed, involving percutaneous epididymal sperm aspiration (PESA), testicular sperm aspiration (TESA) and testicular sperm extraction (TESE). However, for NOA patients, it seems difficult to extract enough testicular sperm through traditional TESE or TESA surgery. It was not until 1998 that Schlegel (Reference Schlegel1999) introduced a new technology called microdissection testicular sperm extraction (micro-TESE), which can achieve a higher sperm retrieval rate (SRR) for NOA patients than traditional methods. In this study, 128 NOA patients underwent micro-TESE surgery on OPU day, and then the retrieved sperm were used to perform ICSI insemination for their spouses’ oocytes, followed by embryo culture and transfer. The SRR, the fertilization rate, embryo development and ART outcome were statistically analyzed in depth. Finally, a systematic evaluation of the clinical treatment effects of micro-TESE surgery combined with ICSI regimen in the treatment of NOA patients was launched.
Materials and methods
Study design
In total, 128 infertile couples underwent micro-TESE surgery combined with an ICSI regimen at our Reproductive Medicine Center from June 2018 to November 2021. All male patients were diagnosed as NOA and met the following inclusion criteria (World Health Organization, 2001; Lu et al., Reference Lu, Huang and Lü2010): no sperm through microscopic examinations after centrifugal precipitation in at least three routine semen examinations; no recent urinary tract infection or reproductive system infection; and no contraindications, excluding those with OA, hypogonadotropic syndrome, hyperprolactinemia, deletion of AZFa or AZFb and spousal ovarian failure. These NOA patients were divided into three groups [the Klinefelter syndrome (KS) group, idiopathic NOA and the secondary NOA groups] based on their own etiologies. In the secondary NOA group, patients carried or had a history of cryptorchidism, inflammation caused by virus infection, and so on. Before entering the treatment cycle, all infertile couples received full interviews and signed informed consent forms.
Ovulation induction regimen
All female patients in this study received our reproductive centre’s routine agonist or antagonist regimen for ovulation induction. The detailed process has been described in our previously published literature (Zhang et al., Reference Zhang, Wang, Hao, Panhwar, Chen, Zou, Ji, Chen, Zhou, Zhao and Cao2017). In the process of inducing follicle development, when two or three follicles with a diameter of ≥18 mm appeared in the ovary, 10,000 IU of human chorionic gonadotropin (hCG; Pregnyl; AESCA Pharma, Austria) was administered to the patient. After 36–38 h, oocytes were retrieved under the guidance of transvaginal ultrasound.
Micro-TESE and sperm collection
Micro-TESE surgery was performed under general anaesthesia on OPU day in parallel. After full disinfection, a 3-cm midline scrotal incision was made to approach the testis. The tunica albuginea of the testis was incised in the mid-testis around the circumference of the testis. In this process, great care was taken to avoid damage to blood vessels. Subsequently, the seminiferous tubules were exposed after controlling bleeding and spreading open the testis. The dilated and opaque tubules (Figure 1) were removed under ×20 magnification (S88 microscope; Zeiss, Germany), shredded and searched for sperm under ×100–150 magnification (SZ800; Nikon, Japan) until three or four sperm (Figure 2) or no sperm were found by exploring the entire testis (the whole process is shown in Video S1). The retrieved tissues containing sperms were sent to the in vitro fertilization (IVF) laboratory for further processing. If no sperm were found, donor semen was prepared.

Figure 1. Seminiferous tubule from patient with NOA during micro-TESE surgery. With the help of a microscope, seminiferous tubules were visible during the surgery. The seminiferous tubules appears dilated and opaque.

Figure 2. Sperm from patients with NOA by micro-TESE surgery. Sperm with normal morphology were retrieved by micro-TESE surgery. Red arrows indicate sperm.
Sperm preparation
First, the sperm sample was placed in a 15-ml conical tube dispensed with 0.5 ml of 40% gradient stock solution underlaid by 0.5 ml of 80% gradient stock solution (Sperm GradTM, VitroLife, Gotebor, Sweden). After 20 min of centrifugation at 800 g, the supernatant was discarded, and the pellet under an 80% layer was picked up. Subsequently, the collected pellet was rinsed with 3 ml of sperm washing medium (Sperm Rinse™, VitroLife, Gotebor, Sweden) by centrifugation for 10 min at 622 g. After removing the supernatant, the pellet at the bottom of the tube was resuspended in 0.1 ml of sperm medium containing pentoxifylline (Sigma, USA) and 5% HSA. Finally, the processed sperm sample underwent 1–2 h of culture in a 37°C and 5% CO2 incubator for subsequent ICSI insemination. For donor sperm, each semen sample was processed using the density gradient centrifugation method for conventional IVF sperm in our centre.
Inseminating, embryo culture and cryopreservation
ICSI insemination in this study was performed by a specific embryologist with 10 years of ICSI operational experience. The collected mature oocytes for each patient underwent ICSI insemination with the retrieved sperm or the donor sperm, and then those injected oocytes underwent 3 days of cleavage embryo culture and 2–3 days of blastocyst culture. On day 5 or day 6 after OPU, the formed high-quality blastocysts (Figure 3) were collected and cryopreserved in −196°C liquid nitrogen using the vitrification method. The scoring criteria for embryos were based on the Tomás and Gardner grading standards (Tomás et al., Reference Tomás, Orava, Tuomivaara and Martikainen1998; Gardner et al., Reference Gardner, Lane, Stevens, Schlenker and Schoolcraft2000), in which a blastocyst with >3BB grade on day 5 or >4BB on day 6 was scored as a high-quality blastocyst. The details involved in ICSI insemination, embryo culture and vitrification have been described in our previously published literature (Ding et al., Reference Ding, Wang, Li, Chen, Zou, Ji, Hao, Xue, Zou, Wei, Zhou, Cao and Zhang2020).

Figure 3. High-quality blastocysts from sperm retrieved by micro-TESE surgery. Representative image of human high-quality blastocysts from NOA patients using an inverted microscope at ×200 magnification (SZ800: Nikon, Japan). The scoring criteria for high-quality blastocysts is based on the Gardner grading standards. Grading of the embryos: (a) 4AA; (b) 4BA; (c) 4BB.
Embryo transfer and pregnancy determination
The cryopreserved blastocysts were warmed according to the Kuwayama protocol and transferred into the uterus with the thickness of endometrium >8 mm in the thawing cycle. The biochemical pregnancy was confirmed if the serum hCG value was ≥25 IU/l after 2 weeks; the clinical pregnancy was confirmed with a sign of the presence of a gestational sac by transvaginal ultrasonography after 28–30 days. The detailed process of embryo warming in this study was described in our previously published literature (Hao et al., Reference Hao, Chen, Zhang, Zhang, Liu, Zhou, Wei, Xu, He, Xing, Lv, Ji, Chen, Zou, Wu, Liu and Cao2020; Li et al., Reference Li, Mu, Elshewy, Ding, Zou, Chen, Chen, Wei, Cao, Zhou and Zhang2021).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software. The quantitative data were expressed as the means ± standard deviation (SD) for numeric variables. The categorical data were expressed as proportions (%). The differences for variables were tested using the t-test, the chi-squared test and the Kruskal–Wallis one-way analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant.
Results
Basic clinical indicators and sperm retrieval results in NOA patients
The 128 patients included 37 cases (28.91%) with Klinefelter syndrome (KS), 68 cases (53.12%) with idiopathic NOA, and 23 cases (17.97%) with the secondary NOA group. There was no significant difference in the levels of T, prolactin (PRL), and estradiol (E2) among the three groups. As shown in Table 1, the testicular volume of the KS group was smaller than that of the other two groups; the SRRs of the KS, idiopathic and the secondary NOA group were 48.65% (18/37), 33.82% (23/68) and 73.91% (17/23), respectively. In addition, the FSH and the LH levels of the KS group were significantly higher than those of the other two groups (all P < 0.05), and the bilateral SRR was higher than that of the secondary NOA group (P < 0.05).
Table 1. Basic clinical indicators and sperm recovery results in NOA patients with different etiologies
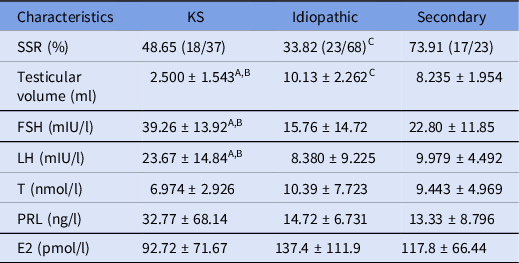
E2: estradiol; FSH: follicle-stimulating hormone; LH: luteinizing hormone; PRL: prolactin; SSR: sperm extraction rate; T: testosterone.
Data analyzed using Student’s parametric t-test, one-way ANOVA or chi-squared test, and presented as means ± standard deviation (SD).
A Statistical difference between the KS group and the idiopathic group, P < 0.05.
B Statistical difference between the KS group and secondary group, P < 0.05.
C Statistical difference between the idiopathic group and the secondary group, P < 0.05.
Comparison of basic clinical indicators between the retrieved and non-retrieved groups in the NOA patients
According to the results of sperm retrieval, the 128 NOA patients were divided into retrieved and non-retrieved groups. The basic clinical indicators are shown in Tables S1–S3. As shown in Table S1, the T value of the retrieved group was significantly lower than that of the non-retrieved group in the KS NOA patients (P < 0.05). As shown in Table S2, the FSH and LH values of the retrieved group were significantly lower than those of the non-retrieved group in the secondary NOA group patients (both P < 0.05). For the idiopathic NOA patients, there were no significant differences found in age, testicular volume or hormone value between the retrieved and the non-retrieved groups, as shown in Table S3.
Fertilization and early embryo development of the retrieved sperm in NOA patients
Of the 128 NOA patients, 58 obtained sperm by micro-TESE surgery successfully. Subsequently, 601 oocytes were inseminated by ICSI with the retrieved sperms, and 398 oocytes were fertilized. Ultimately, 180 oocytes developed into blastocysts, of which 111 were high-quality blastocysts (the retrieved sperm group). The other 70 patients who did not obtain the sperm completed the following treatment plan with the donor sperms (the non-retrieved sperm group). Here, to make univariate comparisons, we just collected and analyzed the clinical data of the patients who underwent ICSI therapy with the donor sperms. All 511 oocytes received ICSI insemination, 435 oocytes were fertilized, and 256 blastocysts formed, of which 185 were high-quality blastocysts. As shown in Figure 4, the cleavage rate of the retrieved sperm was equivalent to that of the donor sperm, whereas the rate of fertilization, high-quality cleavage, blastocyst and high-quality blastocyst in the retrieved sperm group was greatly lower than those of the donor sperm group with extremely significant differences. All of the high-quality blastocysts formed in this study were cryopreserved in −196°C liquid nitrogen for 3 months, followed by vitrification–warming embryo transfer.

Figure 4. Comparison of fertilization and early embryo development of retrieved sperm and donor sperm groups in non-obstructive azoospermia (NOA) patients. The rates of fertilization, high-quality cleavage, blastocyst and high-quality blastocyst in the retrieved sperm group were greatly lower than those of the donor sperm group with significant differences. The cleavage rate of the retrieved sperm resembled that of the donor sperm. *: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001.
Fertilization and early embryo development of the retrieved sperm among NOA patients with different etiologies
To understand the effects of different etiologies on the fertilization and the development potential of the retrieved sperm, the fertilization and early embryo development of the retrieved sperm in the patients with KS, idiopathic and the secondary NOA were further analyzed. As shown in Table 2, the fertilization rate of the secondary NOA group patients was higher than that of either the KS (P < 0.05) or the idiopathic NOA patients (P < 0.05), whereas the rates of high-quality cleavage embryos or blastocysts showed no statistical difference among the three groups (P > 0.05). In addition, the basic female clinical data are shown in Table S4, and no significant difference was found among the three groups.
Table 2. Comparison of fertilization and early embryo development of the retrieved sperm among the KS, idiopathic and secondary NOA patients

Data analyzed using the chi-squared test.
A Statistical difference between the KS group and secondary group, P < 0.05.
B Statistical difference between the idiopathic group and secondary group, P < 0.05.
Embryo transfer outcomes
To date (until this submission), 66 vitrification-thawing embryo transfer cycles in the retrieved sperm group were carried out. In total, 96 warmed blastocysts were transferred, with an average of 1.5 embryos transferred, and clinical pregnancy was established in 20 cycles. To date, 20 healthy offspring have achieved successful delivery. In the donor sperm group, in total, 52 transfer cycles were performed, of which 71 warmed blastocysts were transferred, with an average of 1.37 embryos transferred, and 34 cycles of clinical pregnancy achieved. To date, 20 healthy offspring have been successfully delivered. As shown in Figure 5, the implantation rate and the clinical pregnancy rate of the retrieved sperm group were lower than those of the donor sperm group, and the differences between the two groups were statistically significant (both P < 0.05). The donor sperm group showed a lower abortion rate and a higher live birth rate than the retrieved sperm group with no significant differences. In addition, a regular postnatal follow-up for these infants was performed, and their physical and mental development was normal.

Figure 5. Comparison of embryo transfer outcomes between the retrieved sperms and the donor sperm groups in non-obstructive azoospermia (NOA) patients. The implantation rate and the clinical pregnancy rate of the retrieved sperm group were lower than those of the donor sperm group, and the differences between the two groups were statistically significant. The donor sperm group showed a lower abortion rate and a higher live birth rate compared with the retrieved sperm group with no significant differences (*: P < 0.05).
For the retrieved sperm group, 66 transfer cycles were divided into the KS, idiopathic and the secondary NOA subgroups for further analysis. Irrespective of the implantation rate or the clinical pregnancy rate, the results revealed that the secondary NOA group subgroup presented the better clinical outcomes (P < 0.05). In addition, there was no significant difference in the abortion rate or the live birth rate among the three subgroups. See Table 3 for all details.
Table 3. Embryo transfer outcomes of the patients with different etiologies in the retrieved sperm group

Data analyzed using the chi-squared test.
A Statistical difference between the KS group and the idiopathic group, P < 0.05.
B Statistical difference between the KS group and secondary group, P < 0.05.
C Statistical difference between the idiopathic group and secondary group, P < 0.05.
Discussion
Azoospermia is a serious reproductive health problem caused by male factors that involves genetic and congenital (KS, AZF microdeletion, cryptorchidism, etc.) or acquired factors (surgery, medication, lifestyle, environmental pollution, etc.) (Flannigan et al., Reference Flannigan, Bach and Schlegel2017; Tharakan et al., Reference Tharakan, Luo, Jayasena and Minhas2021). NOA accounts for ∼60% of patients with azoospermia (Adelman et al., Reference Adelman, Lolo, Birkbak, Murina, Matsuzaki, Horejsi, Parmar, Borel, Skehel, Stamp, D’Andrea, Sartori, Swanton and Boulton2013). In fact, in most patients with NOA it is hard to recover or rescue spermatogenesis with drug use only. Patients with NOA had been considered to be sterile until the advent of ICSI (Devroey et al., Reference Devroey, Liu, Nagy, Goossens, Tournaye, Camus, Van Steirteghem and Silber1995). To obtain more sperm of good quality and more embryos with developmental potential, it has been realized gradually that TESE combined with ICSI is recommended as first-line treatment for patients with NOA.
It has been reported that SRR by micro-TESE of the general population of NOA patients was 46.0% (range 20–70.8%) (Verza and Esteves, Reference Verza, Esteves, Nagy, Varghese and Agarwal2019; Achermann et al., Reference Achermann, Pereira and Esteves2021). In our study, in total, 128 NOA patients were performed micro-TESE surgery, of which 58 patients (45.31%, 58/128) had sperm successfully obtained and subsequent ICSI insemination was undertaken. The SRR was roughly consistent with the above research. It was indicated that micro-TESE surgery can improve the SRR of NOA patients. Compared with TESA or the traditional testicular sperm extraction method, the advantages of micro-TESE are clearly the exposure for a small incision and more delicate and targeted examination under an operating microscope with less possible complications (Esteves and Varghese, Reference Esteves and Varghese2012). The variances in the SRR may be attributed to the selection of patients with NOA caused by different factors. As our study showed, the SRRs were 48.65% (18/37), 33.82% (23/68) and 73.91% (17/23) for patients with KS, idiopathic NOA, or secondary NOA, respectively. KS is the most common cause of NOA, with sex chromosome abnormity. A meta-analysis suggested that performing TESE/micro-TESE in KS patients resulted in SRRs of almost 50% (Corona et al., Reference Corona, Pizzocaro, Lanfranco, Garolla, Pelliccione, Vignozzi, Ferlin, Foresta, Jannini, Maggi, Lenzi, Pasquali and Francavilla2017). Patients with secondary NOA with the highest SRR among the three groups may reflect the remaining spermatogenesis function, whereas the SRR of idiopathic NOA patients is the lowest. Actually, SRR in micro-TESE surgery is related to the degree of spermatogenesis impairment. Therefore, micro-TESE surgery can effectively help NOA patients obtain their own sperm hidden in the testis. How to select the correct patients with NOA for micro-TESE surgery and guarantee the success rate of sperm retrieval is an ongoing question. This may be aided to an extent by analysis of the different causes.
Furthermore, based on a common consideration, the SRR of NOA patients may be related to testicular volume, or the values for T, PRL, FSH, LH and E2, and the patient’s age (Chiba et al., Reference Chiba, Enatsu and Fujisawa2016). Tsujimura (Reference Tsujimura2007) reported that the FSH value could be used as a predictor of sperm extraction. The expected SRR in older patients could be significantly reduced, and high levels of FSH and LH in serum may result in sperm extraction failure. However, the results of this study indicated that there was no correlation between SRR and various hormone values or testicular volumes if the aetiology was not considered. Subsequently, further analysis was carried out on NOA patients with different etiologies. The T level of the KS NOA patients in the retrieved group was significantly lower than that of patients in the non-retrieved group, which was not different in the idiopathic NOA patients. For the secondary NOA patients, compared with the non-retrieved group, the levels of FSH and LH in the retrieved group were significantly lower. Osaka et al. (Reference Osaka, Iwahata, Kobori, Shimomura, Yoshikawa, Onota, Yamamoto, Ide, Sugimoto and Okada2020) conducted a study on 52 patients with NOA cryptorchidism and found that the serum LH and FSH levels of the successful sperm extraction group significantly decreased, which was consistent with our data. These results indicated that the SRR of NOA patients with different etiologies is related to certain specific factors.
This study revealed that there was no statistical difference in clinical pregnancy rate among KS, idiopathic and secondary NOA patients. Although the number of MII oocytes in the three groups was different, after comparing the basic data of the patients, it was revealed that there was no statistical difference in their ages and sex hormone levels. Therefore it could be believed that the NOA patients in the three groups could be compared. This result is consistent with Vahidi’s study, except for the different fertilization rate (Vahidi et al., Reference Vahidi, Horoki, Talkhooncheh, Jambarsang, Marvast, Sadeghi and Eskandarian2021). This suggested that the quality of embryos formed with sperm obtained by micro-TESE surgery from NOA patients of different causes is similar. In the subsequent clinical treatment, the patients who did not retrieve sperm through micro-TESE surgery used donor sperm for the following ICSI insemination. The results showed that fertilization, embryo development and clinical outcomes of the donor sperm group were obviously superior to those of the retrieved sperm group, which means that the developmental potential of the retrieved sperm was not as good as that of the donor sperm. However, the utilization of sperm retrieved through micro-TESE surgery still achieved a high-quality blastocyst rate per cycle of 29%, a clinical pregnancy rate of 45.45% and a live birth rate of 20.83%. This brings great hope for NOA patients to have their own biological children. Therefore, micro-TESE surgery combined with ICSI insemination is the most effective treatment regimen for NOA patients. In addition, a further analysis that referred to a variety of indexes during the embryo culture process was performed for the clinical pregnancy and embryo implantation rates. The secondary NOA group presented with an optimal clinical result (compared with the KS NOA group: 68.42% vs 30.77%, 55.56% vs 22.50%; and the idiopathic NOA group 68.42% vs 40.00%; 55.56% vs 28.57%). Although there was no significant difference in the live birth rate among the three groups, the secondary group still had the highest level. Therefore, compared with the other two etiologies of the NOA patients, the retrieved sperm in the secondary group showed an optimal development potential, providing an idea for the future clinical treatment of NOA patients.
In conclusion, micro-TESE surgery combined with ICSI insemination is the most effective treatment regimen for NOA patients. The SRR of NOA patients with different etiologies is related to the spermatogenesis function of the testis; micro-TESE surgery seems the ideal and only option to have biological children.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S096719942200051X
Authors contribution
ZGZ and BLC revised the manuscript and designed study. KJW and DDT collected samples and wrote manuscript. QZ, JW, DDY, and HY collected data. JP and DD edited the manuscript. QSW and YXC analyzed data. All authors read and approved the final manuscript.
Financial support
This study was supported by the General project of National Natural Science Foundation of China under Grant No. 82071724; and the National Science Foundation for Young Scientists of China under Grant No. 82001516.
Conflict of interest
The authors declare that they have no competing interests.
Ethics standards
This study was approved by the Ethics Committee of Anhui Medical University (Hefei, China; Approval No. 20200080). All participants were informed and signed informed consent regarding the risks of surgery. We thank all participants and all authors are grateful to patients who allowed video recording.











