As the second most common neurodegenerative disorder, Parkinson’s disease (PD) is characterized by the classic motor symptoms of tremor, rigidity, bradykinesia and postural instability, thought to be associated with increased mortality compared with the general populations. Previous studies have revealed varied standardised mortality ratios (SMRs) in patients with PD in both clinical and population-based cohorts. SMRs have been found to range from 0.80 to 3.50.Reference Ben-Shlomo, Head and Lees 1 - Reference Ishihara, Cheesbrough, Brayne and Schrag 11 Especially in the post-levodopa era, the SMR from a few investigations have been found to demonstrate a trend to “super-normal” survival rather than excess mortality.Reference Auyeung, Tsoi and Mok 3 - 12 Except for a recent report from Chinese Hong Kong,Reference Auyeung, Tsoi and Mok 3 there is no SMR data from mainland China where levodopa has been used extensively for at least two decades. Hence, confirming the SMR and associated factors with death would be particularly beneficial for this country with a particularly large PD population. In fact, such analysis of mortality in PD patients will be valuable in understanding the natural course of PD and planning the distributions of health resources in China in the present and in the future.
We previously reported a series of cross-sectional clinical studies for motor symptoms and non-motor symptoms among hospital-based clinically definite PD patients.Reference Wang, Hong and Cheng 13 - Reference Chen, Chen and Kang 15 In the present study we used the same databases to identify SMR and factors associated with death.
Methods
Participants
All patients who have visited the movement disorder clinic at the Department of Neurology, Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, and are permanent residents in Shanghai, were recruited consecutively into the present study in 2006. This movement disorder clinic is the biggest referral centre for PD in Shanghai. Patients were referred from general practitioners, family physicians and emergency department doctors. As one of biggest clinical centers in China, the numbers of the patients with PD who visited our clinic annually numbered about five hundred before 2007. However, over half of the patients were from outside of Shanghai metropolis and not permanent residents. When they passed away, their residential city, rather than Shanghai metropolis, recorded information of their death certificates. The control populations for calculating SMR were also limited to the permanent residents of Shanghai. Patients who lived outside of Shanghai metropolis were excluded from the present study. The consecutive patients were identified with the UK PD Brain Bank criteria for the diagnosis of idiopathic PD. Exclusion criteria included a history of dementia or hallucinations within one year of onset of disease, features leading to a diagnosis other than idiopathic PD, or incomplete clinical information. All patients lived in the urban community of Shanghai and were sufficiently competent in Chinese to complete assessments. All participants were assessed at baseline, and 157 were followed in the clinic until December 31, 2011. Four individuals declined to participate in the five year follow up.
The study was approved by the Research Ethics Committee, Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China. Written informed consent was obtained from each participant.
Clinical assessment
At baseline, face-to-face interviews were conducted to collect subjects’ demographic information, living conditions, past and family medical history (especially cognitive impairment, hypertension, diabetes mellitus, hyperlipidemia, other co-morbidities, and medications, etc.), psychiatric history (depression, anxiety, insomnia, neurosis, etc.). Functional independence was assessed with caregivers’ information and Activities of daily living (ADL) scores. The neurologists conducted their clinical assessment by applying the Hoehn and Yahr (H-Y) staging scale, the Mini-mental State Examination (MMSE), the Non-motor Symptoms Scale (NMSS), the Parkinson’s Disease Sleep Scale (PDSS), the Epworth Sleepiness scale (ESS), the Geriatric Depression Scale (GDS), and the Hamilton Anxiety Scale (HAMA). In order to evaluate the relationship between survival and dopaminergic treatment, the daily levodopa equivalent dose (LDE) was calculated as described in a previous study.Reference Wang, Cheng and Zheng 16 All data were gathered and screened for validity by senior movement disorders specialists.
Ascertainment of vital status
In January 2012, we confirmed the status of all patients. For those who had died, death certificates (cause and date of death) were obtained from the database of the Shanghai Municipal Center for Disease Control and Prevention, China. Diagnoses on the death certificate were registered using the international Classification of Disease ninth and tenth revision (ICD-9 and ICD-10). The primary causes of death were grouped into five different categories based on the ICD-9 classifications. 17
Statistical Analysis
All data analyses were performed using SPSS statistical software. SMRs and corresponding 95% confidence intervals (CI) were calculated as the ratios of the observed to the expected number of deaths for selected causes. The expected number was obtained by applying the age- and sex-specific mortality rates of the Chinese urban population in the period 2008-2012 18 to the cohort under study. SMRs were calculated separately for the two genders, and overall. A Kaplan-Meier Survival Curve was used to assess the cumulative survival rate of PD patients.
Descriptive statistics were computed, the t-test or χ2 test were used to calculate differences between groups in continuous and categorical variables, respectively. To explore the potential associated factors for survival, factors with significant difference between two groups with P<0.05 were entered into binary variable logistic regression models that included age and sex and we identified the associated factors for death after adjusting for sex and age.
Results
Sample Characteristics
Among the 157 participants, 92 (58.6%) were males and 65 (41.4%) were females; the mean (+/− standard deviation (SD)) age, in years, for males was 65.48±9.37 and for females, 65.46±11.58; the mean (SD) age in years at onset for males: 60.79±10.23 and for females: 59.04±12.10 (P>0.05)). The average disease duration at baseline is 5.47±0.35 years (Mean ± standard error (SE)). The average H-Y stage was 1.96±0.7 (Mean±SD), and average MMSE score was 26.31±3.59(Mean±SD). Patients with a MMSE score <24 were 11.5% (18/157) of the sample at baseline. Education more than 12 years was attained by 51.6% (81/157) of the subjects. Patients able to maintain the ability of independent living made up 82.8% (130/157) of the population studied. The LDE among the patients was 339.75±18.10 (Mean ±SE)mg/day and there was a positive association between H-Y stage and LDE (P<0.01). An ESS score of over 10 was found to be present in 5.7% (9/157) of PD patients at baseline. Of these, 44.6% (70/157) patients had at least one additional chronic disease such as hypertension or diabetes.
SMR, cause of death and survival duration
A total of eleven (7%) patients died, of whom six (3.8%) died of pneumonia, two (1.3%) died of digestive disorders, one (0.6%) died of cardiac attack, one (0.6%) died of stroke, and one died suddenly with unknown cause at the endpoint. Standardised mortality ratios was only 0.62 (95% CI 0.32 to 1.07, P=0.104) comparable with an age- and sex-matched Chinese urban population over the same period. Even though the findings were based on small numbers, the relative risks were particularly high for death from pneumonia (SMR=2.86) and from digestive disorders (SMR=4.64) (Table 1). Additionally, the survival durations between independent living and dependent living, rather than male and female, longer education and low education, are significantly different (P<0.01) (Figure).

Figure Survival of 157 patients with PD. A: The survival durations of the total sample. B: Different survival between dependent (2) and independent living (1) patients with PD.
Table 1 Standardized mortality ratios and corresponding 95% confidence intervals calculated as the ratios of the observed to the expected number of deaths for selected causes

Abbreviations and symbols: SMR=standardized mortality ratio; CI=corresponding 95% confidence interval.
* The expected number was obtained by applying to the cohort under study the age- and sex-specific mortality rates of the Chinese urban population (resource from: China Public Health Statistical Yearbook (2008-2013))
Related factors of death
The risk factors associated with death are shown in Table 2. Age at onset, independent living, MMSE score, PDSS score and ESS scores were factors that produced statistically significant differences between the survival group and the deceased group (P<0.05).
Table 2 Baseline Characteristics for survival and death patients with PD

PD=Parkinson’s disease, SD=standard deviation, H-Y=Hoehn & Yahr, LDE=levodopa equivalent dose, MMSE=Mini Mental State Examination, NMSS=Non-motor Symptoms Scale, GDS=Geriatric Depression Scale, HAMA=Hamilton Anxiety Scale, ESS=Epworth Sleepiness Scale, PDSS=Parkinson’s Disease Sleep Scale.
The odds of associations before and after adjustment for age and sex between groups are shown in Table 3. In both models, low MMSE score and high ESS score were identified as predictive factors.
Table 3 Baseline factors associated with death adjusted for age and sex
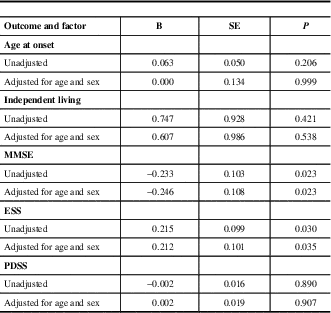
Multivariable association between survival patients and death patients by logistic regression
B=Regression coefficient, SE=Standard error, MMSE=Mini Mental State Examination, ESS=Epworth Sleepiness Scale, PDSS=Parkinson’s Disease Sleep Scale.
Further, we examined the potential predictive effect of daytime somnolence on mortality, after adjusting for the effect of cognitive impairment. We found that there was a negative correlation between ESS score and MMSE score (P<0.05 and we identified ESS as a predictive factor after adjusting MMSE (B=0.013, SE=0.005, P=0.007).
Discussion
Previously, few studies focused on assessing survival in PD cohorts from follow-up studies in China. In the present study, we identified a super-normal survival of patients with PD to the control populations over five years follow-up and also identified risk factors for poor survival, including low MMSE score and high ESS score and we found that cognitive impairment and excessive daytime sleepiness are potential predictors of death.
SMR and cause of death
Although some studies report survival time, SMRs allow comparison between population groups with different demographics and life expectancies.Reference Ishihara, Cheesbrough, Brayne and Schrag 11 The SMR of this cohort was 0.62 (95% CI 0.32 to 1.07), implying that the five year mortality ratio of the patients with PD was not significantly higher than the ratio of the general urban Chinese population. The SMR in the present study is consistent with the survival data from the highly selected subjects in the DATATOP study (eight years of follow-up SMR=0.80) 12 and an Austrian study carried out by Diem-Zangerl and his co-workers (five years of follow-up SMR=0.6; 10 years of follow-up SMR=0.9),Reference Diem-Zangerl, Seppi and Wenning 4 and very close to the previous investigation from Hong Kong (10 years follow-up SMR=1.1).Reference Auyeung, Tsoi and Mok 3
With respect to differences of the follow-up periods and ethnicities, we believe that there are several reasons why our cohort had a better survival compared to the prior studies. Firstly, the selection criteria and diagnostic accuracy in the present study ensure the samples were comprised of idiopathic PD rather than Parkinsonism with atypical features, therefore the recruited patients had a better prognosis and longer survival time. In recent years, the majority of the movement disorder specialists in China have regularly adopted the Chinese guideline for the diagnosis and treatment of PD and have significantly improved their ability to make the diagnosis and use best practice in the management of PD;Reference Chen, Chen, Xiao, Wang and Chen 19 Secondly, clinically based samples may benefit from input from movement disorder specialists compared with community-ascertained cases, including optimal treatment and psychological counselling. Over the last two decades medications available in urban China have almost approached the level found in Western countries, including complex levodopa preparations, non-ergot dopamine agonists, monoamine oxidase B typed (MAO-B) inhibitors, catechol-O-methyltransferase (COMT) inhibitors and so on.Reference Wang, Cheng and Zheng 16 , Reference Chen, Chen, Xiao, Wang and Chen 19 Thirdly, the relatively short follow-up period may be expected to lower the SMR compared to previous surveys with more than ten years of observation.Reference Diem-Zangerl, Seppi and Wenning 4 , Reference Posada, Benito-Leon and Louis 7 Some cohort studies have suggested that SMRs will increase with time.Reference Diem-Zangerl, Seppi and Wenning 4 , Reference Hely, Morris, Traficante, Reid, O’Sullivan and Williamson 5 , Reference Hely, Morris, Reid and Trafficante 20 Therefore it is likely that our cohort would have a higher SMR if observed over a period longer than five years.
Similar to most previous studiesReference Mylne, Griffiths, Rooney and Doyle 6 , Reference Pennington, Snell, Lee and Walker 21 pneumonia (6/11) was the most common cause of death, followed by digestive disorders (peritonitis, and gastrointestinal bleeding, 2/11), acute coronary syndrome (1/11), stroke (1/11), and sudden death of unknown cause (1/11). It is important to note that no malignancy occurred,Reference Diem-Zangerl, Seppi and Wenning 4 - Reference Pennington, Snell, Lee and Walker 21 which has a mortality rate/100000 of about 174 in the normal Chinese urban population. 18 Accounting for over 50% of the deaths, pneumonia is associated with limited mobility, swallowing impairment, and reduced ability to expectorate.Reference Pennington, Snell, Lee and Walker 21 , Reference Monteiro, Souza-Machado, Pinho, Sampaio, Nobrega and Melo 22 However, apart from the current studies, digestive disorders are seldom reported as a cause of death. Non-motor symptoms such as dysfunction of the gastrointestinal system including dysphagia, impaired gastric emptying, decreased bowel movement frequency, and difficulty defecating occur during any stage of PD and may increase the risk of subsequent chronic infection and inflammation in the gastrointestinal tract.Reference Chen, Xu, Wang and Chen 23 Such theories need to be further tested. Additionally, we found that there are different survival durations between dependent and independent living patients with PD. By contrast, the survival durations between male and female, high education and low education, show no significant difference. High Quality of life (QoL) may have the benefit of increasing survival for patients with PD.
Associated factors as predictor of death
The present study shows that low MMSE score at baseline was related to an increased risk of death among PD patients (B=−0.246,SE=0.108, P=0.023 95 % CI: 0.63-0.97). The results are in accordance with the previous observations, which indicated that PD patients with cognitive impairment have a particularly high risk of mortalityReference Auyeung, Tsoi and Mok 3 , Reference Posada, Benito-Leon and Louis 7 , Reference Hughes, Ross, Mindham and Spokes 9 and cognitive impairment is an independent predictor of decreased survival in PD.Reference de Lau, Verbaan, Marinus and van Hilten 24 , Reference Xu, Gong, Man and Fan 25 It is well-known that cognitive impairment, especially Parkinson’s disease dementia (PDD), occurs in 24% to 31% of PD patients,Reference Aarsland, Zaccai and Brayne 26 and both reduced cholinergic activity in the cortex and the spread of fibrillar α-synuclein (α-syn) pathology are thought to be the crucial mechanisms responsible for the development of PDD.Reference Chen, Xu, Wang and Chen 23 , Reference Irwin, Lee and Trojanowski 27 Furthermore, the neocortical α-synuclein pathology may confer a worse prognosis. Our results support the common hypothesis for PD with cognitive impairment as a predictor of death. The molecular pathological mechanism needs to be addressed in more detail in the future.
In the present study, 5.7% of the study participants with ESS score >10 had regular or frequent excessive daytime sleepiness (EDS), one of most common symptoms.Reference Chen, Zhao and Zhang 28 We found that a high ESS score at baseline was associated with an increased risk of death among PD patients (B=0.212, SE=0.101, P=0.035 95% CI: 1.02-1.51), which has been rarely reported from previous similar studies. As a frequent complaint among PD patients, EDS was found to be present in 5.6% of PD patients at baseline, 22.5% at four years, and 40.8% at eight years.Reference Gjerstad, Alves, Wentzel-Larsen, Aarsland and Larsen 29 The etiology of EDS in PD may be the disease process, medications, or other sleep disorders. EDS may be related to dopaminergic medications and is more commonly associated with dopamine agonists than levodopa.Reference Chen, Xu, Wang and Chen 23 One recent study suggested that EDS was associated with an increased risk of dementia,Reference Zhu, van Hilten and Marinus 30 and EDS may indirectly increase risk of mortality by effect of dementia. There also existed a potential predictive effect of daytime somnolence on mortality, after adjusting for the effect of cognitive impairment, in the present study. Before our study, there were no direct reports of a relationship between EDS and mortality in PD. Regarding primary prevention of EDS through regular exercise and strategically planned naps etc., may potentially lead to a decrease in mortality. Additionally, some interesting possible factors, including age at onset, independent living, and PDSS score showed potential with P value <0.05 in the present study. However, there were no significant differences between the two groups by a double variable logistic regression analysis.
Limitations
Our study is limited by its clinical based samples with a relatively short follow-up period, which has the potential for selection bias, attenuating the power to assess the influences of associated factors on death. Consecutive follow-up, over ten or twenty years, is planned. Secondly, we assessed sleep disorders, including EDS, only by ESS rather than polysomnography. Furthermore, the factors associated with death reported here are values taken at baseline and do not take into account possible changes in some factors over time. Therefore, future prospective studies should investigate mortality with a longer follow up period and more sophisticated analytical methods.
Conclusions
This study confirms the similar survival of patients with PD to the control population up to a follow-up of five years. However, cognitive impairment, and EDS at the baseline are associated with death. Interventions tailored to potential risk factors associated with death may offer further benefits.
Acknowledgements
This study was granted by the National Program of Basic Research of China (2011CB504104) and the National Natural Science Foundation (81371407). The authors thank patients, doctors and nurses in the clinic who were involved in the study. We thank Dr. Jeremy Stern at Department of Neurology, St. George’s Hospital and Medical School, London, United Kingdom, for his proof-reading. We also thank Prof. Anthony E Lang at Morton and Gloria Shulman Movement Disorders Center, Toronto Western Hospital, Ontario, Canada, for critical reading and comments on this manuscript.
Disclosures
The authors do not have anything to disclose.






