Introduction
Obstructive sleep apnea (OSA) is a chronic disease characterized by periods of airway closure during sleep due to reduced pharyngeal dilator muscle tone. Reference Eckert and Malhotra1 Positive airway pressure (PAP) is currently the gold standard for OSA therapy, but its positive outcomes are limited by low patient adherence to therapy. Reference Sullivan, Issa, Berthon-Jones and Eves2–Reference Aurora, Casey and Kristo5 Due to the various drawbacks of current OSA therapeutics, new treatment options are required.
Cannabinoids (i.e., cannabis, cannabidiol [CBD], tetrahydrocannabinol [THC]) are a class of drugs which act on the CB1 and CB2 endocannabinoid receptors and have been shown to improve ventilatory stability and reduce apneic events in rodent models. Reference Carley, Paviovic, Janelidze and Radulovacki6–Reference Calik and Carley8 Cannabinoids may achieve these effects via vagal nerve suppression which prevents serotonin-induced apneas. Reference Carley, Paviovic, Janelidze and Radulovacki6,Reference Calik and Carley7,Reference Calik, Radulovacki and Carley9,Reference Calik and Carley10 A synthetic form of THC known as dronabinol was shown to improve sleep apnea by acting on the CB1 and CB2 receptors. Reference Calik and Carley8
The data on how cannabinoids affect OSA and other sleep disorders in humans is currently limited. An initial investigation was conducted on the function of endocannabinoids in 20 humans with OSA. Reference Jumpertz, Wiesner and Blüher11 The study revealed that patients with OSA had higher levels of an endocannabinoid known as oleoylethanolamide (OEA) compared to healthy controls, which suggested cannabinoids may serve a neuroprotective role in sleep apnea. Reference Jumpertz, Wiesner and Blüher11 In a proof-of-concept study in 17 patients with moderate to severe OSA, dronabinol caused a significant reduction in OSA severity after 21 days and no significant adverse events were observed. Reference Prasad, Radulovacki and Carley12 In another study, 73 patients were randomly assigned to receive either 2.5 mg or 10 mg of dronabinol, or placebo. Reference Carley, Prasad and Reid13 Treatment with dronabinol reduced OSA severity and daytime sleepiness but had no effect on the maintenance of wakefulness test (MWT), gross sleep architecture, or overnight oxygenation. Reference Carley, Prasad and Reid13 Because these two studies had small sample sizes and only examined one form of synthetic THC, the effect of cannabis on OSA severity and sleep architecture remains inconclusive.
While cannabinoids may hold therapeutic potential for sleep disorders, it is important to note the broader implications of recreational cannabis use and multi-substance use on sleep architecture and quality. To begin with, cannabinoid use has been linked to polysubstance use and illicit substance use disorders when initiated during adolescence, Reference Fergusson, Boden and Horwood14,Reference Volkow, Baler, Compton and Weiss15 and the use of cannabis, alcohol, and smoking have been associated with insomnia and sleep disturbances in adolescents. Reference Phiri, Amelia, Muslih, Dlamini, Chung and Chang16,Reference Kwon, Park and Dickerson17 Furthermore, cannabinoid use has been shown to induce psychotic-like symptoms, such as thought disorder, delusions, and visual disturbances, Reference Malone, Hill and Rubino18 and is thought to be a risk factor for the development of schizophrenia. Reference Andréasson, Allebeck, Engström and Rydberg19 This is notable because psychosis has been associated with sleep disorders. Reference Reeve, Sheaves and Freeman20 Withdrawal from heavy cannabinoid use has been shown to cause sleep disturbances, including reduced total sleep time, REM sleep, and worsened sleep efficiency, wake after sleep onset, and periodic limb movements, Reference Bolla, Lesage and Gamaldo21 with sleep disturbances peaking within the first week of abstinence. Reference Budney, Moore, Vandrey and Hughes22
Cannabinoids have also been shown to affect sleep architecture. Animal studies and small studies in humans have shown that cannabinoid use may be associated with increased SWS and decreased REM sleep. Reference Pivik, Zarcone, Dement and Hollister23–Reference Pava, Makriyannis and Lovinger26 Some evidence suggests cannabinoid use reduces sleep onset latency, and there is mixed evidence on how it affects time spent in SWS and REM sleep. Reference Babson, Sottile and Morabito27
We performed a retrospective analysis of clinical, physiological, and polysomnographic data from adults who underwent overnight sleep studies at the Sunnybrook Health Sciences Centre Sleep Laboratory between January 1, 2010 and June 9, 2022. Our aim was to explore whether self-reported cannabis use was associated with changes in OSA severity and sleep architecture.
Methods
Objectives
The primary objective of this analysis was to explore whether cannabis use was associated with a reduction in OSA severity, as measured by the respiratory disturbance index (RDI). The RDI was defined as obstructive apneas, hypopneas with desaturation ≥ 4%, and respiratory event-related arousals per hour of sleep. Secondary objectives included assessing whether cannabis use was associated with changes in the following sleep metrics: OSA severity as measured by the apnea–hypopnea index (AHI, defined as obstructive apneas and hypopneas with oxygen desaturation ≥ 4% per hour of sleep), as well as the respiratory arousal index (RAI), total sleep time, sleep efficiency, wake after sleep onset, number of awakenings, sleep onset latency, the proportion of total sleep spent in each sleep stage, RDI and AHI during REM and non-REM sleep, lowest oxygen saturation, and the periodic limb movement index.Reference Boulos, Jairam, Kendzerska, Im, Mekhael and Murray 28 Additionally, the Epworth Sleepiness Scale (ESS) total scores were investigated to gauge how cannabis use affected daytime sleepiness.
We conducted two analyses: (i) a retrospective analysis of data from all patients in our dataset and (ii) a retrospective analysis involving the subset of patients who had complete comorbidity and medication information. In an exploratory subgroup analysis, we also assessed whether there would be an association between cannabinoid use and our sleep metrics of interest in the subset of patients who had moderate to severe OSA (AHI ≥ 15).
Study design
Sleep and medication data were retrospectively collected for all consecutive adults at Sunnybrook Health Sciences Centre who completed diagnostic overnight polysomnography from January 1, 2010 to June 9, 2022. Medication lists were recorded by a sleep technologist during the night of the sleep study. Cannabinoid use of any type or formulation (e.g., CBD and/or THC in liquid, solid, or inhaled form) was recorded based on self-reporting during discussions of medication use with the patient. We excluded patients who underwent a therapeutic study (e.g., used any form of PAP therapy, oral appliances, adaptive servo-ventilation, or split night studies) as well as those who were younger than 18 years of age and those with missing medication logs.
As previously described, participants underwent level 1 in-laboratory polysomnography (Compumedics Neuroscan, Australia) monitored by a technologist using conventional scoring and recording methods. Reference Boulos, Murray and Muir29,Reference Berry, Gamaldo and Harding30 Sleep was manually staged using criteria delineated by the American Academy of Sleep Medicine. Reference Berry, Gamaldo and Harding30
Statistical analysis
Analysis 1: analysis of all patients (2010–2022)
Descriptive statistics were used to characterize the cannabis users vs. the non-cannabis users. Normally distributed continuous variables were reported as means and standard deviations (SD), non-normally distributed continuous variables were reported as median and interquartile range (IQR), and categorical variables were reported as frequency counts. Cannabis users were compared to non-cannabis users using t tests and Mann–Whitney U tests for normally and non-normally distributed continuous variables, respectively. Chi-square tests were used to compare frequency count variables.
Multivariable linear regression models were constructed to investigate the relationship between cannabis use and sleep-related parameters. Sex, age, and BMI were included as covariates in the regression analysis for the minimally adjusted model.
Analysis 2: fully adjusted regression (2010–2015)
In the fully adjusted model, comorbidities, medications, and other substances known to impact OSA severity were included as covariates in addition to sex, age, and BMI. This included a history of hypertension, Reference Shamsuzzaman, Gersh and Somers31 chronic lung disease, Reference Soler, Gaio and Powell32 diabetes, Reference Reutrakul and Mokhlesi33 stroke, Reference Shahar, Whitney and Redline34 depression, Reference Garbarino, Bardwell, Guglielmi, Chiorri, Bonanni and Magnavita35 anxiety, Reference Garbarino, Bardwell, Guglielmi, Chiorri, Bonanni and Magnavita35 as well as the use of sedatives (i.e., benzodiazepines, Reference Pagel and Parnes36 non-benzodiazepine hypnotics, Reference Carter and Eckert37 and barbiturates Reference Pagel and Parnes36 ), opioids, Reference Rosen, Aurora and Kirsch38 and smoking. Reference Wetter, Young, Bidwell, Badr and Palta39 These data were only available for patients that conducted sleep studies between 2010 and 2015.
Prior to modeling, multicollinearity was tested for all variables (tolerance statistic value < 0.4). If multicollinearity was confirmed, only one of the correlated variables was retained in the model. Residual plots were used to assess the final model for any violations to the assumptions of linear regression modeling.
For all analyses, a p-value < 0.05 was considered statistically significant. All data analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY).
Ethical considerations
This retrospective analysis was approved by the Sunnybrook research ethics board (study# 3095).
Data availability statement
To protect the privacy of patients included in this study, the data are not available publicly. The data are available through a data transfer agreement with the corresponding author (MIB).
Results
A total of 10,761 patients aged 18 years and older underwent a sleep study between January 1, 2010, and June 9, 2022. After excluding patients who had missing data or underwent therapeutic polysomnography, 6,958 patients remained; 71 of these were self-reported cannabis users (1.0% of our study sample; Fig. 1). Demographic and polysomnographic data are summarized in Tables 1 and 2, respectively. Cannabis users had a younger overall age than non-cannabis users (Table 1).
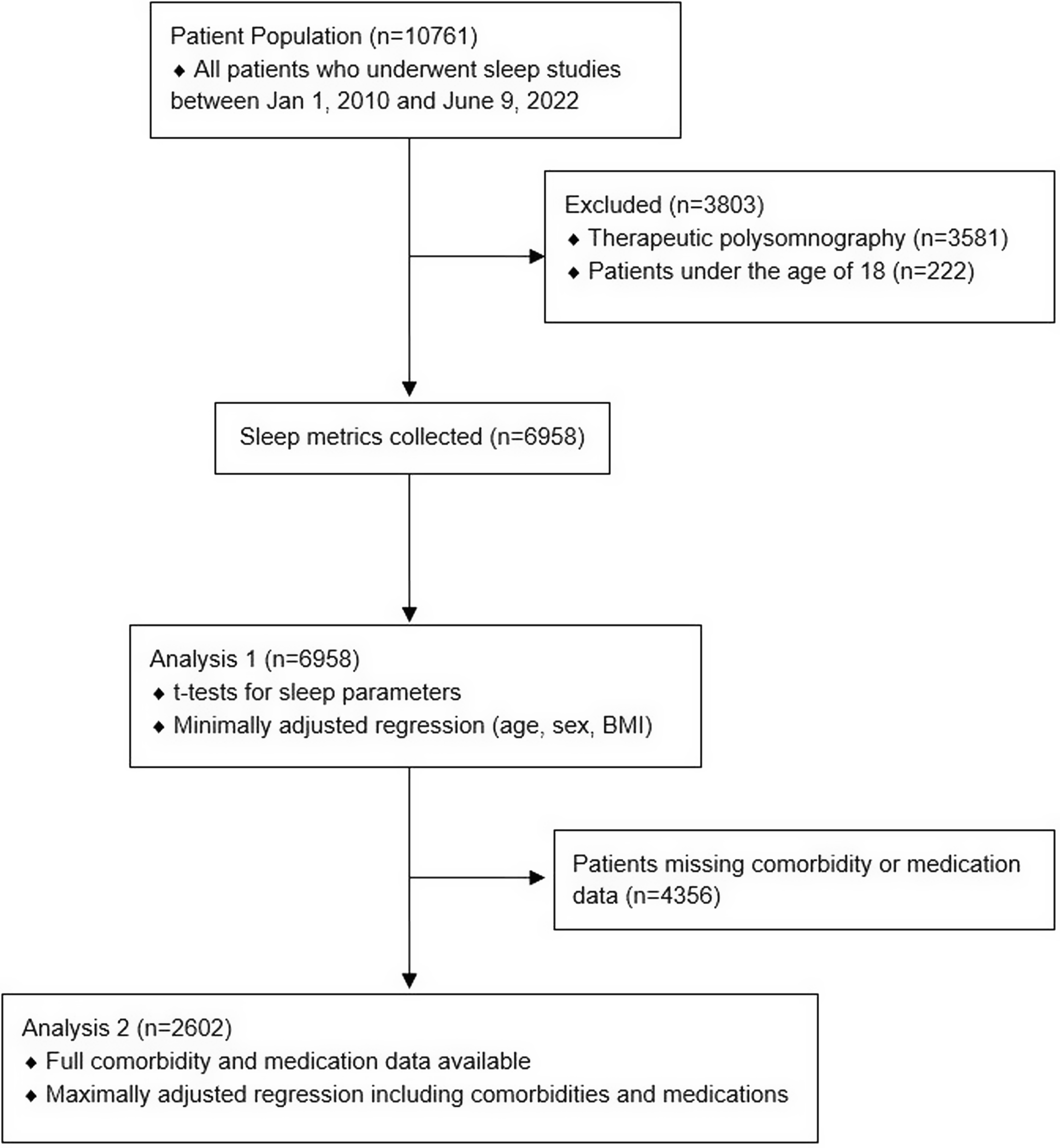
Figure 1: Flow diagram for patients included in analyses.
Table 1: Demographic information of patients who underwent polysomnography comparing cannabis users to non-cannabis users
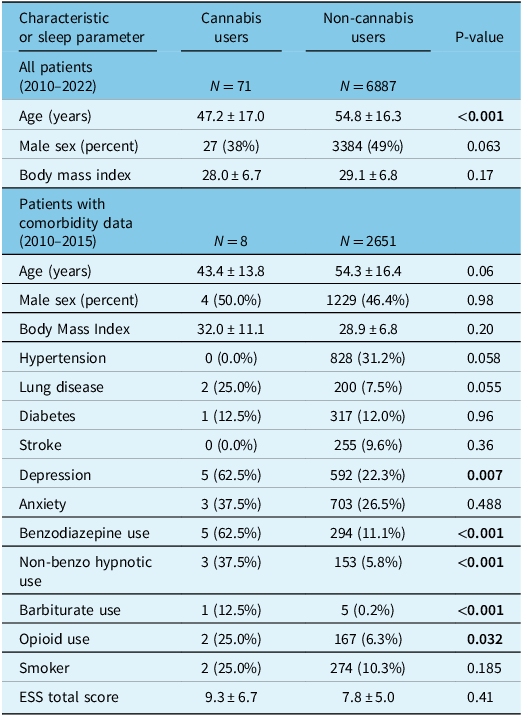
Continuous variables are reported as mean ± standard deviation. ESS = Epworth Sleepiness Scale. Statistically significant P-values (<0.05) have been bolded. All variables with significant P-values in this table were controlled for in the maximally adjusted linear regression models.
Table 2: Sleep parameters of patients who underwent polysomnography comparing cannabis users to non-cannabis users
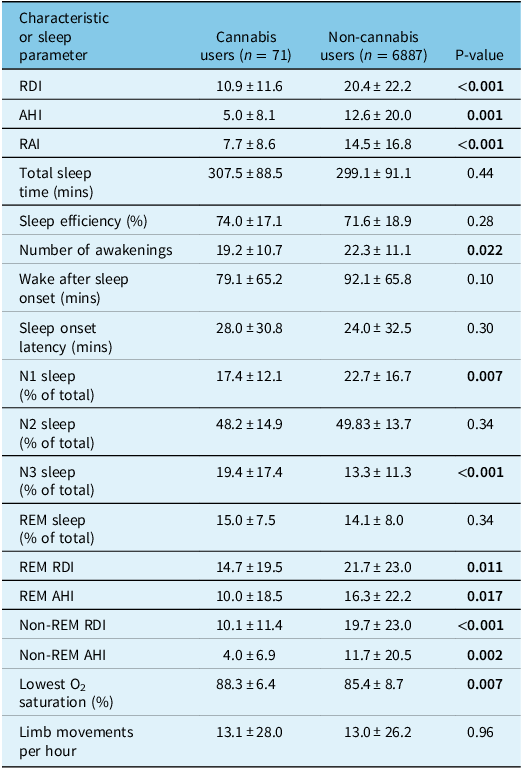
Continuous variables are reported as mean ± standard deviation. RDI = respiratory disturbance index, AHI = apnea–hypopnea index, RAI = respiratory arousal index, REM = rapid eye movement. Statistically significant P-values (<0.05) have been bolded.
For analysis 1 (our minimally adjusted linear regression model), which included 6,958 patients who conducted sleep studies between January 1, 2010 and June 9, 2022, we adjusted for sex, age, and BMI (Table 3). Cannabis use (n = 71) was associated with a reduced RDI (β: −4.8 [95% CI: −9.4, −0.2]; p = 0.042) and RAI (β: −3.7 [95% CI: −7.3, −0.1]; p = 0.046) but did not reach statistical significance for AHI (β: −3.8 [95% CI: −8.0, 0.4]; p = 0.077). In this model, cannabis use was also associated with an increased percentage of N3 stage sleep (β: 4.0 [95% CI: 1.5, 6.5]; p = 0.001). When analyzing only the subset of patients who had moderate to severe OSA (n = 1,728, Table 4), cannabis use was significantly associated with the percentage of stage N3 sleep (β: 11.3 [95% CI: 4.0, 18.6]; p = 0.002) and the ESS total score (β: 12.0 [95% CI: 2.5, 21.6]; p = 0.013).
Table 3: Multivariable linear regression models examining the association between cannabis use and sleep parameters
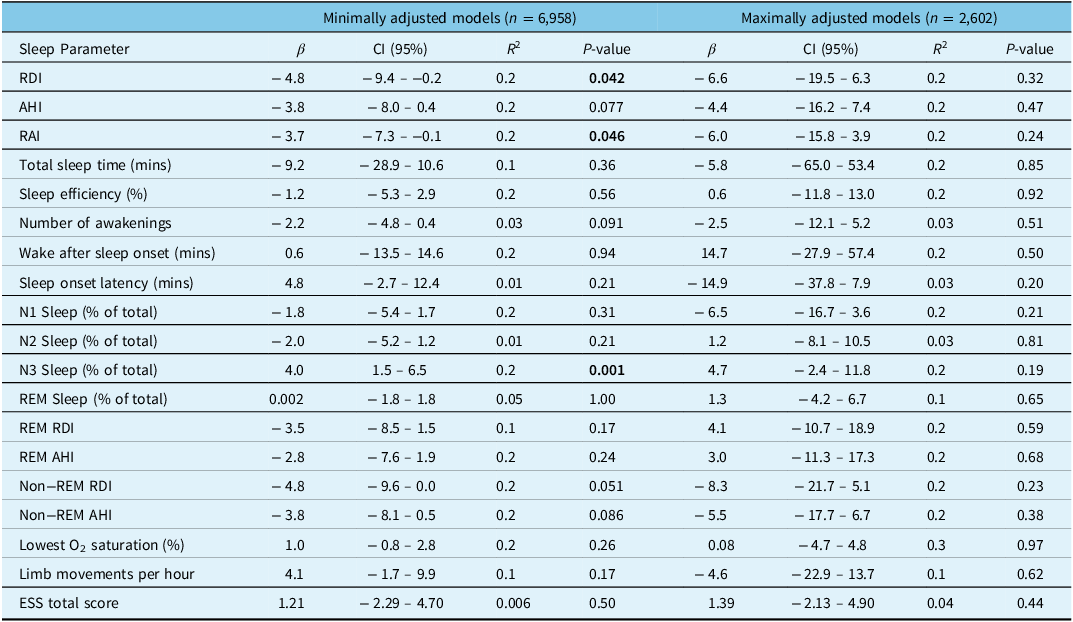
RDI = respiratory disturbance index, AHI = apnea–hypopnea index, RAI = respiratory arousal index, REM = rapid eye movement, ESS = Epworth Sleepiness Scale. Statistically significant P-values (<0.05) have been bolded.
Table 4: Multivariable linear regression models examining the association between cannabis use and sleep parameters in patients with moderate–severe OSA

RDI = respiratory disturbance index, AHI = apnea–hypopnea index, RAI = respiratory arousal index, REM = rapid eye movement, ESS = Epworth Sleepiness Scale. Statistically significant P-values (<0.05) have been bolded.
Analysis 2 (our maximally adjusted linear regression model) included 2,602 patients who completed sleep studies between January 1, 2010 and December 31, 2015 and had additional comorbidity and medication data available (Table 3) After adjusting for covariates, cannabis use (n = 8) was not associated with RDI (β: 6.6 [95% CI: −19.5, 6.3]; p = 0.32), AHI (β: −4.4 [95% CI: −16.2, 7.4]; p = 0.47), or percentage of stage N3 sleep (β: 4.7 [95% CI: −2.4, 11.8]; p = 0.19). When analyzing only patients with moderate to severe OSA (n = 613, Table 4), cannabis use was significantly associated with increased stage N3 sleep (β: 33.5 [95% CI: 15.6, 51.4]; p < 0.001) and decreased REM sleep (β: 16.0 [95% CI: 0.3, 31.7]; p = 0.046), but there was no association with RDI (β: −7.4 [95% CI: −51.2, 36.3]; p = 0.74) or AHI (β: −0.3 [95% CI: −43.7, 43.2]; p = 0.99). In this same subgroup, during REM sleep cannabis use was also associated with reduced RDI (β: 67.1 [95% CI: 11.3, 122.9]; p = 0.018) and AHI (β: 68.5 [95% CI: 12.6, 124.4]; p = 0.016) but there were no associations with changes in RDI (β: −20.4 [95% CI: −67.1, 26.4]; p = 0.39) or AHI (β: −13.3 [95% CI: −59.8, 33.2]; p = 0.57) in non-REM sleep (Table 4). Lastly, in this subgroup of patients with moderate to severe OSA, cannabis use was significantly associated with the ESS total score (β: 9.6 [95% CI: 0.0, 19.2]; p = 0.049).
Discussion
Our study explored the relationship between any self-reported cannabis use and changes in OSA severity and sleep architecture. We found that cannabis users had a younger overall age than the non-cannabis users, but age was controlled for in all of our linear regression models. Our minimally adjusted model showed an association between cannabis use and a reduced RDI and RAI, as well as an increased percentage of stage N3 sleep. In patients with moderate to severe OSA, only the association with N3 stage sleep was statistically significant. Our maximally adjusted model showed that when controlling for comorbidities and medication use (in addition to age, sex, and BMI), there were no significant associations between markers of OSA severity and cannabis use. However, in patients with moderate to severe OSA, this model demonstrated an association between cannabis use and increased stage N3 sleep and decreased REM sleep. It also showed an association between cannabis use and reduced RDI and AHI during REM sleep.
There are very few studies investigating the link between cannabis use and OSA severity in humans. The previous two studies that examined this association were experimental studies with overall sample sizes of 17 patients in Prasad’s study and 73 patients in Carley’s study. Reference Prasad, Radulovacki and Carley12–Reference Carley, Prasad and Reid13 In contrast, this study was a retrospective cross-sectional study with an overall sample size of 6,958 patients with 71 cannabis users.
Our finding that cannabis use was associated with increased stage N3 sleep and decreased REM sleep is consistent with prior literature. Reference Pivik, Zarcone, Dement and Hollister23–Reference Feinberg, Jones, Walker, Cavness and March25 Cannabinoids have been shown to alter the sleep–wake cycle, specifically by increasing the amount of time spent in slow-wave sleep (SWS). This increase may be dependent on the CB1 receptor, as its direct activation results in longer periods of SWS in mice. Reference Pava, Makriyannis and Lovinger26 Furthermore, the administration of a CB1 receptor antagonist has been shown to fragment SWS in rats. Reference Santucci, Storme, Soubrié and Le Fur40 Similar effects on sleep architecture have also been observed in humans. In an early clinical trial, patients receiving 70 mg/day of THC orally had a significantly higher proportion of total sleep time spent in SWS. Reference Feinberg, Jones, Walker, Cavness and March25 Another study demonstrated that dronabinol administration in 15 patients with OSA increased theta and delta frequencies in sleep. Reference Farabi, Prasad, Quinn and Carley41 A different study in 8 healthy volunteers showed that the combination of THC and CBD reduced the duration of stage 3 sleep but did not affect the duration of stages 3 and 4 combined, which are now known together as stage N3 sleep. Reference Nicholson, Turner, Stone and Robson42 Therefore, cannabis appears to play a role in modulating SWS time. Cannabinoids have also been shown to reduce the time spent in REM sleep. Activation of the CB1 receptor in mice reduces the proportion of time spent in REM sleep and decreases the length of REM periods. Reference Pava, Makriyannis and Lovinger26 Another clinical trial, which used an oral dose of 61 ug/kg of THC per day, also supported these results. Reference Pivik, Zarcone, Dement and Hollister23
Since it is known that apnea is worse in REM sleep, Reference Shea, Edwards and White43–Reference McSharry, Saboisky and Deyoung45 we also investigated the effects of cannabis use on AHI and RDI specifically within REM sleep and non-REM sleep. We found that in patients with moderate to severe OSA, cannabis use was associated with reduced AHI and RDI in REM sleep. This finding suggests that the overall improved RDI in cannabis users in our minimally adjusted model may be due to improvements in respiratory physiology rather than changes to the sleep architecture.
The impact of cannabis use on sleep stages may still be an important aspect of its potential therapeutic effect in OSA patients. Stage N3 sleep is widely known to play a pivotal role in healing, recovery, growth, memory, and immune function. Reference Patel, Reddy, Shumway and Physiology46 However, patients with obstructive sleep apnea–hypopnea syndrome (OSAHS) typically have increased stage N1 sleep and decreased stage N3 and REM sleep. Reference Basunia, Fahmy and Schmidt47 One study found that most of the symptoms in adults with OSAHS, such as excessive sleepiness, unrefreshing sleep, attention difficulties, snoring, and insomnia, are linked with a reduction in stage N3 sleep. Reference Basunia, Fahmy and Schmidt47 Therefore, increasing the proportion of stage N3 sleep may be a potential novel target for symptom alleviation in future therapies for OSA. To explore this hypothesis, we analyzed ESS total scores to determine if they were significantly associated with cannabis use. There was no association in our minimally or maximally adjusted models. However, in the exploratory subgroup analysis in patients with moderate to severe OSA, cannabis use predicted higher ESS scores despite also predicting greater stage N3 sleep. This finding would challenge the hypothesis that greater stage N3 sleep correlates with reduced daytime sleepiness as well as the hypothesis that cannabis alleviates OSA severity. However, it should be noted that these subgroup analyses are exploratory in nature due to the small sample size of cannabis users but warrant further investigation.
Cannabis use may cause a reduction in OSA severity, but the limitations of our experimental design have precluded a robust validation of this relationship. First, although there were 6,958 patients included in this study, only 71 of these patients were self-confirmed cannabis. Furthermore, data on comorbidities and medication use were only available for 2,602 patients, and only 8 of these were cannabis users. Therefore, the power was substantially reduced in the maximally adjusted linear regression models, which may explain the lack of association between cannabis use and OSA severity in these models.
Second, it is likely that some of the non-cannabis users were, in fact, consuming cannabis but had not reported their use. If this is true, it would have introduced a misclassification bias and diluted the actual effect of cannabis use on OSA severity. This limitation is supported by the low prevalence of cannabis use in our data (1.0%) compared to reported rates of use in the general population of Canada, even before legalization (14.8%). Reference Rotermann48 This low prevalence is largely attributable to our data extraction methods. Patients undergoing polysomnography at Sunnybrook were asked about medication use. Therefore, our dataset only identifies cannabis users based on self-reporting during medication-related discussions. It is likely that many patients using cannabis recreationally before its legalization in 2018 were less inclined to self-report cannabis use.
Third, it is possible that only specific formulations and routes of administration for cannabis are effective for reducing OSA severity. The previous two human studies exclusively investigated the effects of dronabinol, an oral synthetic THC medication, but our study included several different formulations of cannabis with different combinations of THC and CBD. These formulations included dronabinol, nabilone (Cesamet), nabiximols (Sativex), and recreational marijuana, consumed in several different routes of administration including orally, sublingually, topically, and inhaled. There may also be a dose-dependent effect of cannabis on OSA severity as previous studies have demonstrated, which we were unable to account for due to a lack of dosing information. Reference Bolla, Lesage and Gamaldo21,Reference Budney, Moore, Vandrey and Hughes22,Reference Nicholson, Turner, Stone and Robson42
In conclusion, our data suggest that cannabis use is not associated with a reduction in OSA severity after controlling for confounding factors such as medical comorbidities and the impact of medication use. It is recommended that future larger studies investigating cannabis use in OSA control for potential confounding variables, as well as examine the impact of cannabinoid formulation, route, and dose on OSA severity. Our data also demonstrated that cannabis use was associated with an increased percentage of N3 stage sleep and decreased REM sleep in patients with moderate to severe OSA; moreover, cannabis use predicted reduced OSA severity during REM in these patients, suggesting improved respiratory physiology.
Pharmacological options are needed for OSA therapy due to the lack of compliance and comfort with PAP therapy, and cannabis could represent a pharmacological therapy option for some patients. This should continue to be investigated in future human studies.
Author contribution
MV: conceptualization, study design, data acquisition, data analysis and interpretation, and writing of the manuscript.
SJ: conceptualization, study design, data acquisition, data analysis, and writing of the manuscript.
PG: data acquisition and writing of the manuscript.
EC: data acquisition.
TK: study design and data analysis.
BJM: data acquisition, interpretation.
MIB: conceptualization, study design, supervision of data acquisition, analysis, and interpretation, and writing of the manuscript.
All authors: critically revised the manuscript for important intellectual content, final approval of the manuscript for publication, and agreed to be accountable for all aspects of the work.
Funding statement
Outside of the submitted work, Dr M. I. Boulos reports in-kind support for his research program from Braebon Medical Corporation. Dr M. I. Boulos also reports financial support for his research program from the Mahaffy Family Research Fund, Green Mountain, Dr Robert Maggisano, Dr Lawrence Cohen, and grant funding from the Canadian Institutes of Health Research, the Division of Neurology at the University of Toronto, the Sunnybrook Education Advisory Council and Education Research Unit, the Alternative Funding Plan from the Academic Health Sciences Centres of Ontario, the Heart & Stroke Foundation of Canada, and the Restless Legs Syndrome Foundation.
Competing interests
Dr Boulos has also received consultancy and speaker fees from Jazz Pharmaceuticals, Paladin Labs, and Eisai. Dr T. Kendzerska reports consulting fees from Pitolisant Medical Advisory Board (Paladin Labs Inc.). Mr. M. Veitch, Mr. S. Jairam, Mr. P. Gurges, Dr E. Cohen, and Dr B. J. Murray report no disclosures.







