INTRODUCTION
Acute gastrointestinal illness (AGI) has a huge public health impact in terms of disease incidence, prevention issues and healthcare costs. The majority of AGI is caused by infectious agents.
Over the last years the burden of AGI has been a field of research in many countries. The methodology for community surveys has improved over the years and a common case definition was established to ensure international comparability [Reference Cantwell1–Reference O'Brien3]. These efforts resulted in cross-sectional studies for estimates for the burden of AGI from various countries [Reference Adlam4–Reference Scavia18] and additionally a few population-based cohort studies [Reference Wheeler19–Reference Tam21].
In Germany, epidemiological analyses of infectious diseases often rely on data from the national notifiable infectious disease surveillance system. Laboratory-confirmed cases are notified to the local public health office. Clearly, these cases represent only the tip of the surveillance pyramid [Reference O'Brien3]. Many infections go unnoticed because not all patients seek medical care. Moreover, although medically indicated, a stool sample is not investigated from all patients for the causative agent, which is prerequisite for notification. Varying degrees of under-ascertainment by age group, sex, socioeconomic status and geographical region can result in biased comparisons of incidence estimates. Despite the fact that many of the gastrointestinal pathogens are notifiable, there is no syndromic surveillance of AGI in Germany. In this situation conducting cross-sectional surveys using a standardized syndromic case definition for AGI is a necessary alternative. These surveys provide representative population estimates of the true burden of acute gastrointestinal disease. A cross-sectional study in North Germany in 2004 verified that in a large proportion of AGI patients an infectious agent could have been detected, indicating that the majority of these are caused by infectious agents [Reference Karsten22]. This is why cross-sectional data on the incidence of AGI can complement the system of notifiable disease surveillance while helping to unravel true differences of disease burden from under-ascertainment and reporting artefacts. Furthermore, data on baseline incidence can help to interpret the data of syndromic surveillance implemented on an ad hoc basis (e.g. in the case of large and widely dispersed outbreaks) [Reference Wadl23]. Additionally incidence estimates may help to formulate precise case definitions differentiating between baseline disease incidence and outbreak case excess during outbreak analyses of gastrointestinal diseases [Reference Buchholz24–Reference Wilking26]. Information on determinants of socioeconomic status, personal health behaviour and self-perceived health status might help to formulate hypotheses on risk factors for related diseases. The data on healthcare utilization of AGI cases is useful for cost-effective analysis of specific interventions.
The main objective of this study was to estimate the incidence of self-reported AGI in the German adult population and investigate sociodemographic and epidemiological factors as determinants of AGI. Furthermore, we also assessed clinical manifestations and utilization of medical services in cases of AGI.
METHODS
Survey methodology
The population-based telephone survey GEDA [Gesundheit in Deutschland Aktuell (Current Health in Germany)] is part of the German health monitoring programme. The GEDA methodology has been described in detail elsewhere [Reference Kroll and Lampert27, Reference Schmich, Häder, Häder and Kühne28]. The sampling population consisted of the resident German-speaking adult population living in private households with a fixed telephone line (landline). The proportion of households having landline access was 89% in 2009. Landlines are more prevalent in households with two or more persons and single households of persons aged > 60 years [29]. Landline access is slightly more prevalent in rural villages and more prevalent in the elderly. The targeted number of respondents was 21 000. The telephone-number sample was created using the Gabler-Häder design [Reference Gabler and Häder30]; it was based on phone numbers taken from public telephone directories. In order to include people with unlisted numbers, random numbers were produced based on German area codes, thereby allowing selection of numbers not registered in directories. Altogether, a number pool consisting of both published and unpublished phone numbers was created. In order to give each element of the population the same theoretical likelihood of being interviewed, an additional selection of target persons was conducted at the household level, using the ‘last-birthday method’. The computer-assisted telephone interview (CATI) method was applied. Interviews were conducted by 205 interviewers in 385 working shifts which were planned to be equally distributed over the study period. To allow the maximum number of people to be contacted the shifts were worked Mondays to Fridays between 16:00 and 20:00 hours and on Saturdays from 14:00 to 18:00 hours. Interviewers were balanced regarding age and sex to avoid interviewer bias. Selected telephone numbers were attempted to be contacted up to 15 attempts. If telephone contact was made with the household, the interviewer determined if the household contained two or more adults and then asked to speak with the adult household member with the most recent birthday. Study participants were enrolled from July 2008 to June 2009, the response was 29·1% and showed little variation by place and time. In order to improve representativeness, survey weights were generated to adjust for deviation of the target population to the German adult population based on estimates of the German Federal Statistical Office of Germany for 2009. This included a design weighting to (i) number of telephone numbers in the household and (ii) number of persons in the household, and additionally a weighting post-stratification to (iii) age, (iv) sex, (v) region and (vi) education (standard classification: ISCED) [Reference Kroll and Lampert27, Reference Schmich, Häder, Häder and Kühne28]. Unless otherwise stated, all statistical analyses account for the weights.
Case definition
Our case definition was based on that proposed by International Collaboration on Enteric Disease ‘Burden of Illness’ Studies [Reference Majowicz2]. It deviates from this as vomiting was defined as having had at least three episodes. Diarrhoea was defined as ⩾ 3 loose stools in a 24-h period. Persons who reported having diarrhoea but then reported having fewer than three loose stools in a 24-h period were considered not to have had diarrhoea. Vomiting was defined as having had at least three episodes on one day. We defined cases of AGI as respondents who either self-reported diarrhoea (n = 1·501) or vomiting (n = 379) in the 4 weeks preceding the interview. All others were defined as non-cases.
We excluded all subjects from the analysis who had chronic gastrointestinal diseases, i.e. Crohn's disease (n = 60), ulcerative colitis (n = 68), stomach cancer and intestinal tumours (n = 31), irritable bowel (n = 138) or coeliac disease (n = 22) or who were pregnant (n = 147). We excluded missing values regarding chronic gastrointestinal diseases (n = 28). Due to data privacy exclusion criteria for alcohol and drugs, related diarrhoea or vomiting could not be included in the study's case definition.
Data analysis
Four-week incidence (I 4 wk) as incidence proportion (expressed in %) was calculated as
, where x k is a binary variable indicating whether a person k was a case or not and where w k is the weight of x k. 95% confidence intervals (CIs) were used as interval estimates. For reasons corresponding to other studies the annual incidence (I annual) was calculated as I annual = I 4 wk × (365/28) and expressed in terms of episodes/person per year. Odds ratios (ORs) as measures of the association between disease determinants as explanatory variables and the defined AGI cases as outcome variable were obtained using logistic regression controlled for age, sex and age × sex interaction. For categorical variables the category with the highest number of participants was chosen as reference but never one of the two extreme categories. Spatial reference is the administrative system of districts in Germany and time reference is the day of the telephone interview. For comparison of the proportion between age groups and sex, the two-tailed P value for the z test from logistic regression was used. For comparison of average means between age groups and sex, the two-tailed P value from linear regression was used. The analyses were performed using Stata v. 12 (Stata Corp., USA). All statistical tests and regression analyses account for the study weights using the ‘svy’ command in Stata v. 12.0 [31].
RESULTS
The response rate was 29·1% and a total of 21 262 interviewees responded to the survey, of which 20 800 were eligible for case definition. Of these 11 761 (58·2%) were female. Median age was 46 years (range 18–100, interquartile range: 35–60 years). Altogether 1562 (7·5%) persons reported an AGI. Incorporating the study weights the I 4 wk of AGI in adults was 7·3% (95% CI 6·9–7·8) corresponding to an I annual of 0·95 episodes/person per year (95% CI 0·90–0·99). Extrapolated to the 2009 overall adult population of 68·3 million this resulted in an estimated 64·9 (95% CI 62·0–67·8) million episodes of AGI per year in adults in Germany.
Effects of age, gender, seasonality, and geographical region
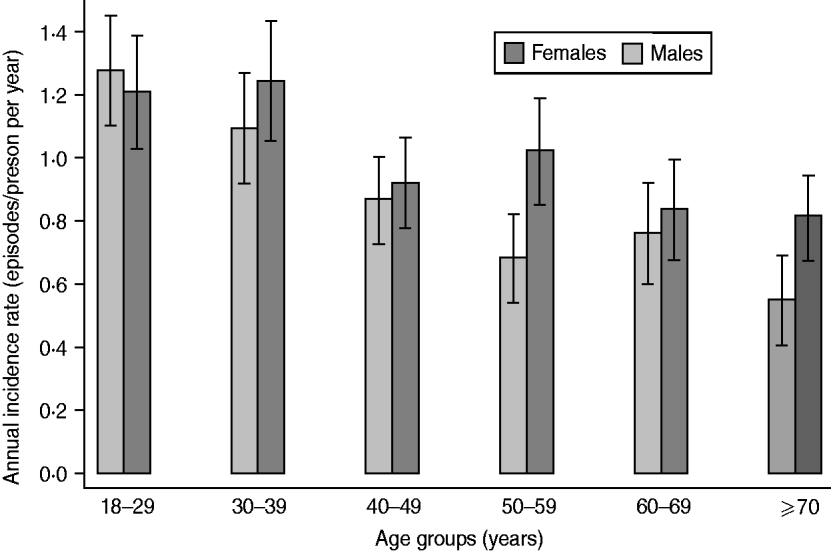
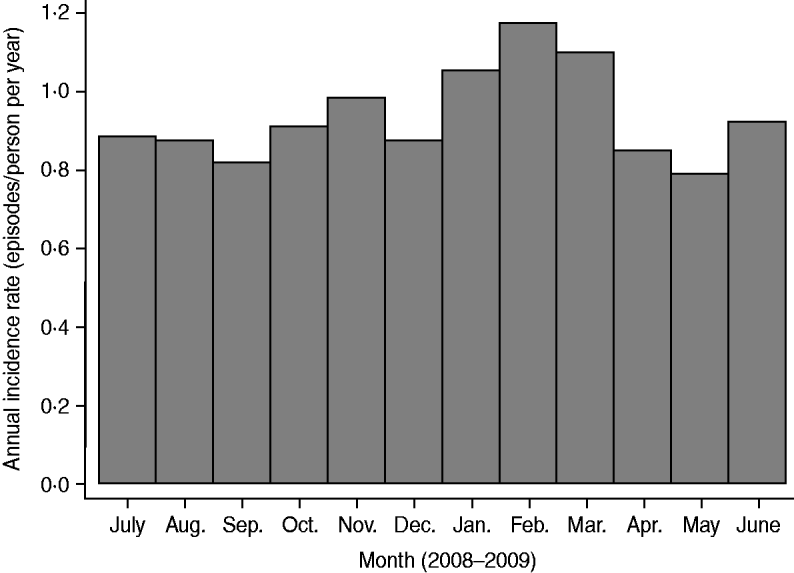
Statistically significant differences of I annual were found between age groups and a borderline significant age × sex interaction (P = 0·055) (Fig. 1). Incidence was highest in young males (I annual = 1·29 episodes/person per year, I 4 wk = 9·9%), and lowest in older males (I annual = 0·54 episodes/person per year, I 4 wk = 4·2%). We observed an overall declining trend of AGI with increasing age both in males (P < 0·001) and females (P = 0·003). Overall, female adults had a higher odds of reporting AGI than males, but this did not reach statistical significance (P = 0·081). However, in the older age groups incidence was higher in females than in males (most pronounced in the 50–59 years age group). Incidence in the Eastern German federal states (former German Democratic Republic plus West Berlin) was slightly lower (I annual = 0·86 episodes/person per year) but this difference in comparison with Western states failed to achieve significance (P = 0·169). The variation of AGI during the survey period is shown in Figure 2. Incidence ranged from 0·79 in May 2008 to 1·17 in February 2009. A peak was observed from January 2009 to March 2009.

Fig. 1. Distribution of annual incidence of acute gastrointestinal illness in Germany in 2008–2009 by age and sex (n = 20 800).

Fig. 2. Timely distribution of annual incidence of acute gastrointestinal illness by month in Germany (n = 20 800). Time reference is the day of telephone interview.
Other risk factors
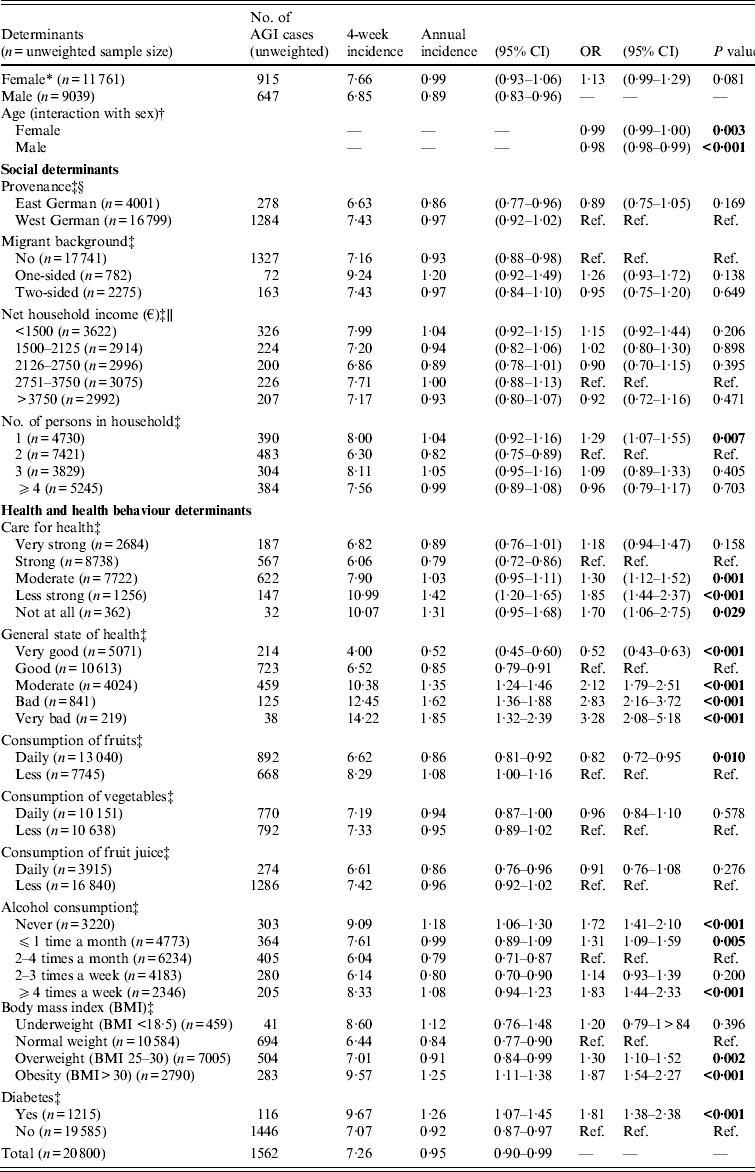
Due to the influence of age and sex of the respondents on the incidence and the odds ratios, all estimates for risk factors were adjusted for these factors and their interaction (Table 1). Generally, social characteristics seemed to be weak predictors of AGI in this study. Migrant status or migration background was not associated with AGI. Compared to a two-person household as a reference category, people living alone were more likely to report AGI (OR 1·29, 95% CI 1·07–1·55), whereas larger households of four or more people (OR 0·96, 95% CI 0·79–1·17) or the number of infants in the household (OR 1·12, 95% CI 0·97–1·29) were not a risk factor.
Table 1. Incidence estimates and determinants for acute gastrointestinal illness (AGI) in adults in Germany, 2009 (weighted) (n = 20 800)

OR, Odds ratio; CI, confidence interval.
* OR adjusted for age.
† In years and reference category 18 years.
‡ Adjusted for age, sex, and age × sex interaction.
§ Whole of Berlin as East Germany.
¶ A person was defined as having a one-sided migrant background if at least one of the parents was not born in Germany, a two-sided migrant background if the person had no German citizenship, moved to Germany after birth or both parents were not born in Germany.
∥ In Euro (€), discretized by quintiles.
Bold values indicate significance.
The overall health and health behaviour were significantly associated with AGI. Based on a self-reported scale from 1 to 5 (where 1 is very good and 5 is very bad), the respondents were asked for their general state of health. An association of AGI and perceived poor general health status was apparent. The question regarding personal health awareness revealed that in comparison to respondents who strongly cared for their health, people with self-reported moderate, poor or very poor personal health awareness more frequently reported AGI. However, respondents who self-reported a very strong care did not benefit. Daily consumption of fruits was inversely associated with AGI (OR 0·82, CI 0·72–0·95), whereas daily consumption of vegetables and fruit juice were not statistically related for AGI.
Alcohol consumption had a two-way association with risk for AGI. Overall, 6234/20 756 (30%) of the interviewees reported drinking alcohol 2–4 times a month. People who reported drinking ⩾ 4 times a week had a significantly higher incidence (OR 1·83, 95% CI 1·44–2·33). By contrast, respondents who reported drinking less than the reference category or who reported never drinking alcohol (OR 1·72, 95% CI 1·41–2·10) were also significantly more at risk. In an analysis of the influence of alcohol consumption stratified on diarrhoea and vomiting, the results are qualitatively the same for AGI as the combined outcome.
The body mass index (BMI) was a significant predictor of AGI. With each increase of BMI score the risk of AGI increased by 5%. The risk for obese persons (BMI > 30) was the highest. Diabetes mellitus was reported by 5·8% of the participants and is significantly associated with AGI in our study (OR 1·81, 95% CI 1·38–2·38).
Symptoms, severity and healthcare utilization
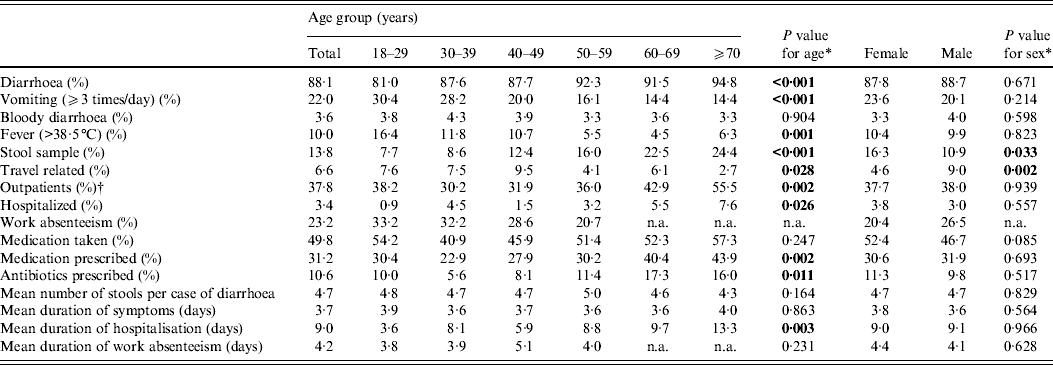
Of the 1562 respondents who met the case definition for AGI, 78·0% reported diarrhoea, 11·9% reported vomiting and 10·1% experienced both symptoms (Table 2). Bloody diarrhoea was reported by 3·6%. Mean duration of illness was 3·7 days without significant differences by age and sex.
Table 2. Proportions and average means for associated factors and medical actions taken of cases of acute gastrointestinal illness by age and sex (n = 1562)

n.a., Not applicable.
* Proportions: two-tailed P value for the z test from logistic regression; means: two-tailed P value from linear regression.
† Either doctor in private practice, medical service in hospital without hospitalization.
Bold values indicate significance.
More than one third (37·8%) of cases sought outpatient medical care and 3·4% were hospitalized. Overall, 13·8% of the cases reported providing a stool sample for microbiological examination. This was significantly more often reported by the elderly (24·4% in those aged > 70 years) and by females (16·3%). There were no significant differences between West Germany and East Germany regarding the proportion of patients reporting an outpatient visit (P = 0·49) and providing a stool sample (P = 0·70). Altogether 49·8% of the AGI cases reported having taken medication against AGI, 31·2% reported a medical prescription and 10·6% of all AGI cases reported antibiotic therapy. The latter was significantly more often prescribed for elderly patients. Fever was associated with antibiotic therapy prescriptions (P < 0·001); however, bloody diarrhoea was not (P = 0·462). In our study, 23·2% of the AGI patients had to stay away from work with a mean duration of work absenteeism of 4·2 days.
In an extrapolation of these proportions to the total adult German population of 68·3 million, the results of our survey add up to 24·5 million outpatient visits, 9·0 million stool sample analyses, nearly 6·9 million antibiotic prescriptions dispensed, 19·9 million days in hospital and 63·2 million working days lost.
DISCUSSION
This is the first survey conducted in Germany which is able to obtain nationwide representative data on the incidence and distribution of AGI in the population. The study, limited to adults, provides nationally representative estimates for disease burden. The sampling procedure and the statistical weighting ensure unbiased samples regarding age, sex, geographical region and education status. For adults young age was the strongest predictor for AGI in this study. It is possible that older persons have been repeatedly exposed to gastrointestinal pathogens during the course of life and acquired relative immunity. Alternatively risky behaviour of young adults leaving home and beginning to prepare their own food (second weaning) could be a contributing factor.
International comparison
Incidence for AGI in adults in Germany is generally in line with observations in similar studies in other countries. Comparisons between countries have to consider the varying case definitions. We used a more restrictive criterion for the identification of AGI-related vomiting. This could explain differences to other countries, e.g. to the Danish study with a higher proportion of cases with vomiting explaining a higher overall incidence [Reference Muller, Korsgaard and Ethelberg15]. Additionally, most of the studies, unlike ours, included children and adolescents and the cut points for age groups vary between the analyses. A decreasing trend for age is reported in all similar studies. From neighbouring European Union member-state countries Denmark reported higher incidence in younger adults but the same in the elderly (from I annual = 2·0 in the 20–29 years age group to I annual = 0·75 in those aged ⩾ 70 years) [Reference Muller, Korsgaard and Ethelberg15], Italy reported lower incidence in the elderly (from I annual = 1·11 in the 10–24 years age group to I annual = 0·33 in those aged ⩾ 75 years) [Reference Scavia18] and Poland almost the same incidence (I annual = 0·9 in the 15–64 years age group) [Reference Baumann-Popczyk6] as in Germany. Furthermore, reported incidence of AGI seem to be generally in the same range as in the USA [Reference Herikstad11], Canada [Reference Sargeant, Majowicz and Snelgrove16], Hong Kong [Reference Ho12] and Australia [Reference Hall10], lower than in New Zealand [Reference Adlam4], Cuba [Reference Aguiar5] and Norway [Reference Kuusi14], respectively, but higher than in Great Britain [Reference Tam21], Malta [Reference Gauci7], Ireland [Reference Scallan17] and Malaysia [Reference Gurpreet9].
Generally, similar proportions of bloody diarrhoea in cases of AGI are reported from Denmark and Canada. Higher levels of bloody diarrhoea are published from New Zealand and Australia and lower levels from studies of the USA, Ireland and Malta.
Seasonality and geography
The distribution of AGI during the 1-year study period can be explained by the seasonal variation in infections with viral enteric pathogens (most prominently norovirus). This assumption is supported by the fact that the peak of AGI in January and February, as observed in the present study, corresponds to the 2009 peak of norovirus activity in Germany in the fourth calendar week which was observed from the available surveillance systems of notifiable disease [32]. By contrast, seasonality of AGI of bacterial origin (most prominently Campylobacter and Salmonella) peaks in August but the summer season is not prominent for AGI incidence in this study. A similar seasonal AGI pattern was discovered by a study in Northern Germany who also found viral pathogens more frequently detected [Reference Karsten22]. In neighbouring countries seasonality is similar [Reference Baumann-Popczyk6, Reference Muller, Korsgaard and Ethelberg15, Reference Scavia18] while other studies report seasonal peaks in summer [Reference Hall10]. There is no difference in the distribution of AGI between East and West Germany which has important implications for the interpretation of surveillance data of enteric diseases in Germany. Since reunification in 1990 a substantial higher incidence in the notification of infectious diseases was observed for East Germany mainly regarding gastrointestinal pathogens [32, Reference Reintjes33]. This increased notification rate is not mirrored by the syndromic level in our survey results which demonstrate no differences between the two parts of the country. Based on our study, higher notification rates in the East are not explainable by different food consumption habits, differing population dynamics or the higher daycare attendance rates in infants as has been hypothesized. Different disease awareness in the population could be an alternative explanation.
Determinants
Social and economic factors are not or only weakly associated with the risk of AGI. Although a differing lifestyle can be assumed, migrants and people with migrant background have no increased risk for AGI. The respondent's self-reported income groups are statistically related to AGI. Thus, in contrast to developing countries, financial factors seem to have a minor influence in industrialized countries like Germany where sanitary hygiene standards and microbiological quality of food and drinking water are not (in the same degree) dependent on socioeconomic status. Larger household size is not related to disease, not even the number of infants. This is surprising, assuming that a large proportion of the AGI cases in this study became infected with pathogens via the faecal–oral route related to contact frequencies with other persons [Reference Karsten22, 32]. However, infants who are more susceptible for faecal–oral transmission were not included in the study. Single-person households with a higher infection rate are a notable exception which could be explained by frequent visits to cafeterias and purchases at fast-food outlets (e.g. takeaways).
Characteristics of health and health behaviour are prominent determinants of AGI. The degree of negative perception of an individual's own health status is linearly correlated with the incidence of AGI. This may reflect the influence of concomitant diseases on gastrointestinal infections. A similar effect could be observed regarding self-reported care for health.
There are conflicting results regarding alcohol consumption in this study. It can be hypothesized that people who frequently drink alcohol, as was asked in our study, also do so excessively and therefore report vomiting and diarrhoea. Additionally, frequent consumption of alcohol could affect the overall immunity of the participants [Reference MacGregor34, Reference Szabo35]. By contrast, people who never drink alcohol are also more likely to report AGI. The reasons for this could be attributed to confounding factors such as alcohol abstinence due to health grounds which also lead to AGI or the defining conditions of diarrhoea and vomiting.
Eating fruit is preventive, drinking fruit juice and eating vegetables is not. This could be a true protective effect or a proxy for nutritional habits. An increase in BMI is associated with AGI. From the probabilistic point of view, eating more in greater quantities and frequency increases the likelihood of consuming a foodborne pathogen as also does a hypothesized increased consumption of risk food. In addition to this effect, eating fat- and carbohydrate-rich and low-fibre diets could have a harmful effect on the gastrointestinal flora and a high BMI is correlated with a generally impaired immunity [Reference Milner and Beck36].
Healthcare utilization
AGI is common in adults in Germany and represents a significant burden of illness. Utilization of healthcare service is high in all age groups. Surveys from other countries that asked about prescription of antibiotics reported less utilization than in our survey in Germany (10·6%). This is despite the fact that we did not include children and adolescents, groups which are known to have higher prescription rates for antibiotic treatment than adults. In Ireland 5·6% of AGI cases self-report antibiotic use [Reference Scallan37], 8·3% in the USA [Reference Scallan37], 3·8% in Canada [Reference Scallan37], 3·6% in Australia [Reference Scallan37], 6·5% in Italy [Reference Scavia18] and 6·4% in New Zealand [Reference Adlam4]. Prescription and consumption practices appear to be considerably different in Germany. It remains to be investigated if this disproportion is caused by disease inherent factors, different diagnostic guidelines or differences in healthcare systems. The higher proportions of faecal sampling for diagnostics in the elderly and in females will presumably result in differences in notification rates of gastrointestinal pathogens. This could explain increased overall incidence of laboratory-confirmed cases of norovirus in females in Germany [32]. Incidence estimates based on notification data of viral pathogens increase in those aged ⩾60 years which was not seen in our study. In the future, differences in age and sex in the distribution of enteric pathogens should be interpreted against this background.
Limitations and strengths
Our study relies on a large number of individuals which generates precise estimates. Persons in households without a landline phone connection could not participate in the study which presumably resulted in underrepresentation of some social groups. This might have introduced selection bias; however, applying the study weights attempts to correct for basic demographic factors. Additionally, the response rate of 29·1% indicates a possibility for selection bias. The study is limited in a way that a full assessment of all possible underlying chronic disease was not able to be performed. The international comparability of incidence estimates is hampered by different survey methodologies and cases definitions. We decided that the AGI-related vomiting criterion requires at least three episodes as we believe that a single episode of vomiting may not be specific enough for an AGI infection. This differs from other work groups. Missing exclusion criteria for alcohol and drugs in the cases definition were related to data privacy and considered by the authors as a minor deviation. Many gastrointestinal symptoms occur as a consequence of primary respiratory infections and this aspect could not be assessed. A future study could benefit from including respiratory symptoms as part of the survey process. The study concerned AGI from all aetiological agents and did not distinguish between bacterial or viral origin. Pathogen-specific risk estimates would provide a better insight into the risk of frequent bacterial gastroenteritis [Reference Gkogka8, Reference Ethelberg38–Reference Scallan40]. The telephone interview did not encompass all assumable risk factors for AGI or even precisely record dietary habits. Instead it focused on some general and partially subjective underlying factors and self-reporting of those might introduce exposure misclassification; this certainly restricts the interpretation of our study.
CONCLUSION
The burden of AGI is high in adults in Germany. Almost 9/10 individuals experience an episode each year. Risk factors are more pronounced on the general state of health and health behaviour than on the social situation. Markedly, high rates of prescribed antibiotics in AGI patients should be further investigated. The health-promoting effect of eating fruits and the prevention of obesity, diabetes and alcohol abuse should be increasingly supported in Germany. This survey should be complemented with children and adolescents in the future.
ACKNOWLEDGEMENTS
This study was partly funded by the European Community Network of Excellence, MedVetNet and the Ministry of Health in Germany. The authors thank M. Höhle for his support and comments on the statistical analyses.
DECLARATION OF INTEREST
None.